Evolution of the cytoskeleton
- PMID: 17563102
- PMCID: PMC2630885
- DOI: 10.1002/bies.20601
Evolution of the cytoskeleton
Abstract
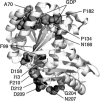
The eukaryotic cytoskeleton appears to have evolved from ancestral precursors related to prokaryotic FtsZ and MreB. FtsZ and MreB show 40-50% sequence identity across different bacterial and archaeal species. Here I suggest that this represents the limit of divergence that is consistent with maintaining their functions for cytokinesis and cell shape. Previous analyses have noted that tubulin and actin are highly conserved across eukaryotic species, but so divergent from their prokaryotic relatives as to be hardly recognizable from sequence comparisons. One suggestion for this extreme divergence of tubulin and actin is that it occurred as they evolved very different functions from FtsZ and MreB. I will present new arguments favoring this suggestion, and speculate on pathways. Moreover, the extreme conservation of tubulin and actin across eukaryotic species is not due to an intrinsic lack of variability, but is attributed to their acquisition of elaborate mechanisms for assembly dynamics and their interactions with multiple motor and binding proteins. A new structure-based sequence alignment identifies amino acids that are conserved from FtsZ to tubulins. The highly conserved amino acids are not those forming the subunit core or protofilament interface, but those involved in binding and hydrolysis of GTP.
(c) 2007 Wiley Periodicals, Inc.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

