Relative quantification of carboxylic acid metabolites by liquid chromatography-mass spectrometry using isotopic variants of cholamine
- PMID: 17563114
- PMCID: PMC2538948
- DOI: 10.1021/ac062416m
Relative quantification of carboxylic acid metabolites by liquid chromatography-mass spectrometry using isotopic variants of cholamine
Abstract
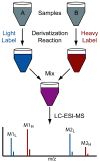
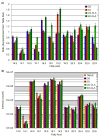
Labeling reagents that differ only in their isotopic composition offer a powerful approach to achieve relative quantification between samples by ESI-MS. Heavy and light isotopic forms of cholamine, which contain a positively charged quaternary ammonium group, were synthesized and tested as new labeling reagents for the relative quantification of carboxylic acid-containing metabolites, specifically fatty acids. The positive charge on cholamine ensures that the labeled product is also positively charged under all LC-MS conditions, regardless of mobile-phase pH. This leads to high ionization efficiency and correspondingly high detection sensitivity, demonstrated here for the analysis of fatty acids in positive ion mode ESI-MS after reversed-phase separation under acidic conditions. Good accuracy and precision were obtained by mixing heavy- and light-labeled hydrolyzed egg lipid extracts in different known ratios. The relative quantification results for 10 observed fatty acids had an average absolute error of 4.6% and an average coefficient of variation (CV) of 2.6%. The labeling strategy yielded a median CV of 6% when employed for fatty acid analysis of eggs from chickens fed various dietary supplements.
Figures

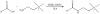
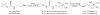
References
-
- Birkemeyer C, Luedemann A, Wagner C, Erban A, Kopka J. Trends Biotechnol. 2005;23:28–33. - PubMed
-
- Rochfort S. J Nat Prod. 2005;68:1813–1820. - PubMed
-
- Sumner LW, Mendes P, Dixon RA. Phytochemistry. 2003;62:817–836. - PubMed
-
- Weckwerth W, Fiehn O. Curr Opin Biotechnol. 2002;13:156–160. - PubMed
-
- Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L. Nat Biotechnol. 2000;18:1157–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

