Real-time analysis of uptake and bioactivatable cleavage of luciferin-transporter conjugates in transgenic reporter mice
- PMID: 17563383
- PMCID: PMC1965515
- DOI: 10.1073/pnas.0703919104
Real-time analysis of uptake and bioactivatable cleavage of luciferin-transporter conjugates in transgenic reporter mice
Abstract
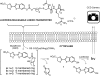
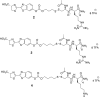
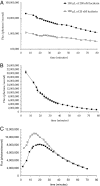
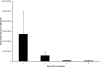
Many therapeutic leads fail to advance clinically because of bioavailability, selectivity, and formulation problems. Molecular transporters can be used to address these problems. Molecular transporter conjugates of otherwise poorly soluble or poorly bioavailable drugs or probes exhibit excellent solubility in water and biological fluids and at the same time an enhanced ability to enter tissues and cells and with modification to do so selectively. For many conjugates, however, it is necessary to release the drug/probe cargo from the transporter after uptake to achieve activity. Here, we describe an imaging method that provides quantification of transporter conjugate uptake and cargo release in real-time in animal models. This method uses transgenic (luciferase) reporter mice and whole-body imaging, allowing noninvasive quantification of transporter conjugate uptake and probe (luciferin) release in real time. This process effectively emulates drug-conjugate delivery, drug release, and drug turnover by an intracellular target, providing a facile method to evaluate comparative uptake of new transporters and efficacy and selectivity of linker release as required for fundamental studies and therapeutic applications.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
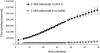

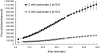
References
-
- Snyder EL, Dowdy SF. Pharm Res. 2004;21:389–393. - PubMed
-
- Trehin R, Merkle HP. Eur J Pharm Biopharm. 2004;58:209–223. - PubMed
-
- Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. ChemBioChem. 2005;6:2126–2142. - PubMed
-
- Gupta B, Levchenko TS, Torchilin VP. Adv Drug Deliv Rev. 2005;57:637–651. - PubMed
-
- Zorko M, Langel U. Adv Drug Deliv Rev. 2005;57:529–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

