Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors
- PMID: 17563384
- PMCID: PMC1890560
- DOI: 10.1073/pnas.0701096104
Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors
Abstract
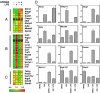
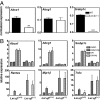
Alzheimer's disease (AD) is an age-dependent neurodegenerative disease that causes progressive cognitive impairment. The initiation and progression of AD has been linked to cholesterol metabolism and inflammation, processes that can be modulated by liver x receptors (LXRs). We show here that endogenous LXR signaling impacts the development of AD-related pathology. Genetic loss of either Lxralpha or Lxrbeta in APP/PS1 transgenic mice results in increased amyloid plaque load. LXRs regulate basal and inducible expression of key cholesterol homeostatic genes in the brain and act as potent inhibitors of inflammatory gene expression. Ligand activation of LXRs attenuates the inflammatory response of primary mixed glial cultures to fibrillar amyloid beta peptide (fAbeta) in a receptor-dependent manner. Furthermore, LXRs promote the capacity of microglia to maintain fAbeta-stimulated phagocytosis in the setting of inflammation. These results identify endogenous LXR signaling as an important determinant of AD pathogenesis in mice. We propose that LXRs may be tractable targets for the treatment of AD due to their ability to modulate both lipid metabolic and inflammatory gene expression in the brain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tontonoz P, Mangelsdorf DJ. Mol Endocrinol. 2003;17:985–993. - PubMed
-
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. Nature. 1996;383:728–731. - PubMed
-
- Repa JJ, Mangelsdorf DJ. Annu Rev Cell Dev Biol. 2000;16:459–481. - PubMed
-
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Nat Med. 2003;9:213–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

