Expression of integrin-linked kinase in the murine lens is consistent with its role in epithelial-mesenchymal transition of lens epithelial cells in vitro
- PMID: 17563721
- PMCID: PMC2765468
Expression of integrin-linked kinase in the murine lens is consistent with its role in epithelial-mesenchymal transition of lens epithelial cells in vitro
Abstract
Purpose: To evaluate the expression and location of integrin-linked kinase (ILK) within the mouse lens and to characterize the role of this protein during mouse lens epithelial cells (LEC) differentiation in vitro.
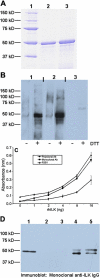
Methods: Transcription levels of ILK mRNA were determined by RT-PCR in cultured cells and lens tissue. ILK protein was detected by immunoblotting, immunocytochemistry, immunohistochemistry, and immunoprecipitation. A role for ILK in the outgrowth of LEC from dissected mouse lens explants was determined by the use of ILK short interfering RNA (siRNA). Affinity-purified polyclonal anti-recombinant human ILK IgG was prepared and characterized for these experiments. A comparison of several anti-ILK antibodies was performed by immunoblotting, immunoprecipitation, and ELISA.
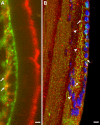
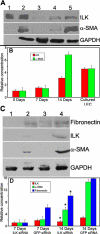
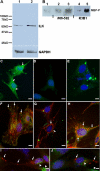
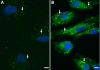
Results: ILK was transcribed in LEC and lens fiber cells in vivo. ILK protein was expressed in the differentiating LEC at the equatorial region of the lens and, to a lesser extent, within the cortical and nuclear fiber cells. LEC in vitro produced copious ILK, which exhibited a filamentous pattern throughout the cytoplasm. The expression of ILK was increased during epithelial-mesenchymal-transition (EMT) of LEC from lens explants, whereas inhibition of ILK by siRNA delayed expression of the EMT markers smooth muscle alpha-actin and fibronectin.
Conclusions: Analysis of ILK expression, localization, and activity in the mouse lens and cultured LEC is substantially facilitated by the generation of a multi-functional, polyclonal, affinity-purified anti-ILK antibody. Expressed in most tissues and cells lines, ILK is unexpectedly restricted to the equatorial LEC and differentiated fiber cells of the mouse lens. The occurrence of ILK expression with LEC differentiation is consistent with the positive regulatory function of ILK, which is revealed in a model of EMT in vitro. This is the first study to show the expression of ILK in the lens and its unique distribution pattern within cultured lens epithelia.
Figures

Similar articles
-
PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase.J Am Soc Nephrol. 2007 Sep;18(9):2534-43. doi: 10.1681/ASN.2007030315. Epub 2007 Jul 26. J Am Soc Nephrol. 2007. PMID: 17656471
-
Inhibition of integrin-linked kinase via a siRNA expression plasmid attenuates connective tissue growth factor-induced human proximal tubular epithelial cells to mesenchymal transition.Am J Nephrol. 2008;28(1):143-51. doi: 10.1159/000110019. Epub 2007 Oct 19. Am J Nephrol. 2008. PMID: 17951996
-
Integrin linked kinase (ILK) is required for lens epithelial cell survival, proliferation and differentiation.Exp Eye Res. 2014 Apr;121:130-42. doi: 10.1016/j.exer.2014.01.013. Epub 2014 Jan 25. Exp Eye Res. 2014. PMID: 24472646
-
Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: a model for cataract formation.Cells Tissues Organs. 2005;179(1-2):43-55. doi: 10.1159/000084508. Cells Tissues Organs. 2005. PMID: 15942192 Review.
-
Integrin linked kinase (ILK) expression and function in vascular smooth muscle cells.Cell Adh Migr. 2009 Apr-Jun;3(2):174-6. doi: 10.4161/cam.3.2.7374. Epub 2009 Apr 10. Cell Adh Migr. 2009. PMID: 19262169 Free PMC article. Review.
Cited by
-
Secreted Protein Acidic and Rich in Cysteine in Ocular Tissue.J Ocul Pharmacol Ther. 2015 Sep;31(7):396-405. doi: 10.1089/jop.2015.0057. Epub 2015 Jul 13. J Ocul Pharmacol Ther. 2015. PMID: 26167673 Free PMC article. Review.
-
Effects of lentiviral RNA interference-mediated downregulation of integrin-linked kinase on biological behaviors of human lens epithelial cells.Int J Ophthalmol. 2016 Jan 18;9(1):21-8. doi: 10.18240/ijo.2016.01.04. eCollection 2016. Int J Ophthalmol. 2016. PMID: 26949605 Free PMC article.
-
Lens Fibrosis: Understanding the Dynamics of Cell Adhesion Signaling in Lens Epithelial-Mesenchymal Transition.Front Cell Dev Biol. 2022 May 17;10:886053. doi: 10.3389/fcell.2022.886053. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35656546 Free PMC article. Review.
-
The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase.J Biol Chem. 2008 Aug 15;283(33):22826-37. doi: 10.1074/jbc.M706563200. Epub 2008 May 23. J Biol Chem. 2008. PMID: 18503049 Free PMC article.
-
Integrin-linked kinase deletion in the developing lens leads to capsule rupture, impaired fiber migration and non-apoptotic epithelial cell death.Invest Ophthalmol Vis Sci. 2012 May 17;53(6):3067-81. doi: 10.1167/iovs.11-9128. Invest Ophthalmol Vis Sci. 2012. PMID: 22491404 Free PMC article.
References
-
- Lee SP, Youn SW, Cho HJ, Li L, Kim TY, Yook HS, Chung JW, Hur J, Yoon CH, Park KW, Oh BH, Park YB, Kim HS. Integrin-linked kinase, a hypoxia-responsive molecule, controls postnatal vasculogenesis by recruitment of endothelial progenitor cells to ischemic tissue. Circulation. 2006;114:150–9. - PubMed
-
- Ishii T, Satoh E, Nishimura M. Integrin-linked kinase controls neurite outgrowth in N1E-115 neuroblastoma cells. J Biol Chem. 2001;276:42994–3003. - PubMed
-
- Belvindrah R, Nalbant P, Ding S, Wu C, Bokoch GM, Muller U. Integrin-linked kinase regulates Bergmann glial differentiation during cerebellar development. Mol Cell Neurosci. 2006;33:109–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
