Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection
- PMID: 17563918
- PMCID: PMC2699425
- DOI: 10.1002/eji.200636751
Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection
Abstract
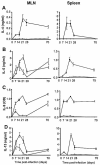
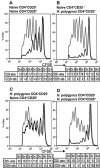
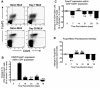
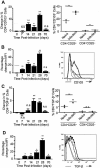
Regulatory T cell responses to infectious organisms influence not only immunity and immunopathology, but also responses to bystander antigens. Mice infected with the gastrointestinal nematode parasite Heligmosomoides polygyrus show an early Th2-dominated immune response (days 7-14), but by day 28 a strongly regulatory profile is evident with antigen-specific IL-10 release and elevated frequency of CD4(+) T cells bearing surface TGF-beta. CD4(+)CD25(+) T cells from infected mice show enhanced capacity to block in vitro effector T cell proliferation. CD4(+)CD25(+) cell numbers expand dramatically during infection, with parallel growth of both CD25(+)Foxp3(+) and CD25(+)Foxp3(-) subsets. CTLA-4 and glucocorticoid-induced tolerance-associated receptor, also associated with regulatory T cell function, become more prominent, due to both expanded CD25(+) cell numbers and increased expression among the CD25(-) population. Both intensity and frequency of CD103 expression by CD4(+) T cells rise significantly, with greatest expansion among CD25(+)Foxp3(+) cells. While TGF-beta expression is observed among both CD25(+)Foxp3(+) and CD25(+)Foxp3(-) subsets, it is the latter population which shows higher TGF-beta staining following infection. These data demonstrate in a chronic helminth infection that Foxp3(+) regulatory T cells are stimulated, increasing CD103 expression in particular, but that significant changes occur to other populations including expansion of CD25(+)TGF-beta(+)Foxp3(-) cells, and induction of CTLA-4 on CD25(-) non-regulatory lymphocytes.
Figures
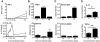



References
-
- Maizels RM, Yazdanbakhsh M. Regulation of the immune response by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2003;3:733–743. - PubMed
-
- Mountford AP, Trottein F. Schistosomes in the skA balance between immune priming and regulation. Trends Parasitol. 2004;20:221–226. - PubMed
-
- Harnett W, Goodridge HS, Harnett MM. Subversion of immune cell signal transduction pathways by the secreted filarial nematode product, ES-62. Parasitology. 2005;130(Suppl):S63–S68. - PubMed
-
- Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor M, Allen JE. Helminth parasites: Masters of regulation. Immunol. Rev. 2004;201:89–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

