Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review
- PMID: 17564490
- PMCID: PMC1891320
- DOI: 10.1371/journal.pmed.0040202
Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review
Erratum in
- PLoS Med. 2007 Aug;4(8):e254
Abstract
Background: The global tuberculosis epidemic results in nearly 2 million deaths and 9 million new cases of the disease a year. The vast majority of tuberculosis patients live in developing countries, where the diagnosis of tuberculosis relies on the identification of acid-fast bacilli on unprocessed sputum smears using conventional light microscopy. Microscopy has high specificity in tuberculosis-endemic countries, but modest sensitivity which varies among laboratories (range 20% to 80%). Moreover, the sensitivity is poor for paucibacillary disease (e.g., pediatric and HIV-associated tuberculosis). Thus, the development of rapid and accurate new diagnostic tools is imperative. Immune-based tests are potentially suitable for use in low-income countries as some test formats can be performed at the point of care without laboratory equipment. Currently, dozens of distinct commercial antibody detection tests are sold in developing countries. The question is "do they work?"
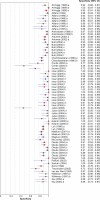
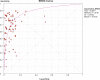
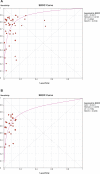
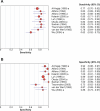
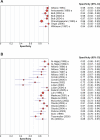
Methods and findings: We conducted a systematic review to assess the accuracy of commercial antibody detection tests for the diagnosis of pulmonary tuberculosis. Studies from all countries using culture and/or microscopy smear for confirmation of pulmonary tuberculosis were eligible. Studies with fewer than 50 participants (25 patients and 25 control participants) were excluded. In a comprehensive search, we identified 68 studies. The results demonstrate that (1) overall, commercial tests vary widely in performance; (2) sensitivity is higher in smear-positive than smear-negative samples; (3) in studies of smear-positive patients, Anda-TB IgG by enzyme-linked immunosorbent assay shows limited sensitivity (range 63% to 85%) and inconsistent specificity (range 73% to 100%); (4) specificity is higher in healthy volunteers than in patients in whom tuberculosis disease is initially suspected and subsequently ruled out; and (5) there are insufficient data to determine the accuracy of most commercial tests in smear microscopy-negative patients, as well as their performance in children or persons with HIV infection.
Conclusions: None of the commercial tests evaluated perform well enough to replace sputum smear microscopy. Thus, these tests have little or no role in the diagnosis of pulmonary tuberculosis. Lack of methodological rigor in these studies was identified as a concern. It will be important to review the basic science literature evaluating serological tests for the diagnosis of pulmonary tuberculosis to determine whether useful antigens have been described but their potential has not been fully exploited. Activities leading to the discovery of new antigens with immunodiagnostic potential need to be intensified.
Conflict of interest statement
Figures


References
-
- World Health Organization. Global tuberculosis control: Surveillance, planning, financing. WHO report. Geneva: World Health Organization; 2007. WHO/HTM/TB/2007.376.
-
- Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–2775. - PubMed
-
- Toman K. What are the advantages and disadvantages of fluorescence microscopy? In: Frieden T, editor. Toman's tuberculosis: Case detection, treatment, and monitoring—questions and answers. 2nd edition. Geneva: World Health Organization; 2004. pp. 31–34.
-
- Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, et al. Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: A systematic review. Lancet Infect Dis. 2006;6:664–674. - PubMed
-
- Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

