Characterization of lamin mutation phenotypes in Drosophila and comparison to human laminopathies
- PMID: 17565385
- PMCID: PMC1885830
- DOI: 10.1371/journal.pone.0000532
Characterization of lamin mutation phenotypes in Drosophila and comparison to human laminopathies
Abstract
Lamins are intermediate filament proteins that make up the nuclear lamina, a matrix underlying the nuclear membrane in all metazoan cells that is important for nuclear form and function. Vertebrate A-type lamins are expressed in differentiating cells, while B-type lamins are expressed ubiquitously. Drosophila has two lamin genes that are expressed in A- and B-type patterns, and it is assumed that similarly expressed lamins perform similar functions. However, Drosophila and vertebrate lamins are not orthologous, and their expression patterns evolved independently. It is therefore of interest to examine the effects of mutations in lamin genes. Mutations in the mammalian lamin A/C gene cause a range of diseases, collectively called laminopathies, that include muscular dystrophies and premature aging disorders. We compared the sequences of lamin genes from different species, and we have characterized larval and adult phenotypes in Drosophila bearing mutations in the lam gene that is expressed in the B-type pattern. Larvae move less and show subtle muscle defects, and surviving lam adults are flightless and walk like aged wild-type flies, suggesting that lam phenotypes might result from neuromuscular defects, premature aging, or both. The resemblance of Drosophila lam phenotypes to human laminopathies suggests that some lamin functions may be performed by differently expressed genes in flies and mammals. Such still-unknown functions thus would not be dependent on lamin gene expression pattern, suggesting the presence of other lamin functions that are expression dependent. Our results illustrate a complex interplay between lamin gene expression and function through evolution.
Conflict of interest statement
Figures

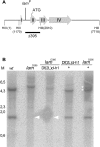
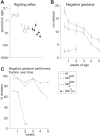
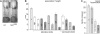

References
-
- Gruenbaum Y, Goldman RD, Meyuhas R, Mills E, Margalit A, et al. The nuclear lamina and its functions in the nucleus. Intermational Review of Cytology. 2003;226:1–62. - PubMed
-
- Shumaker DK, Kuczmarski ER, Goldman RD. The nucleoskeleton: lamins and actin are major players in essential nuclear functions. Curr Op Cell Biol. 2003;15:358–366. - PubMed
-
- Riemer D, Weber K. The organization of the gene for Drosophila lamin C: limited homology with vertebrate lamin genes and lack of homology versus the Drosophila lamin Dmo gene. Eur J Cell Biol. 1994;63:299–306. - PubMed
-
- Riemer D, Stuurman N, Berrios M, Hunter C, Fisher PA, et al. Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. J Cell Science. 1995;108:3189–3198. - PubMed
-
- Stuurman N, Delbecque J-P, Callaerts P, Aebi U. Ectopic overexpression of Drosophila Lamin C is stage-specific lethal. Exp Cell Res. 1999;248:350–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

