Differential in vitro inhibitory activity against HIV-1 of alpha-(1-3)- and alpha-(1-6)-D-mannose specific plant lectins: implication for microbicide development
- PMID: 17565674
- PMCID: PMC1904181
- DOI: 10.1186/1479-5876-5-28
Differential in vitro inhibitory activity against HIV-1 of alpha-(1-3)- and alpha-(1-6)-D-mannose specific plant lectins: implication for microbicide development
Abstract
Background: Plant lectins such as Galanthus nivalis agglutinin (GNA) and Hippeastrum hybrid agglutinin (HHA) are natural proteins able to link mannose residues, and therefore inhibit HIV-target cell interactions. Plant lectins are candidate for microbicide development.
Objective: To evaluate the activity against HIV of the mannose-specific plant lectins HHA and GNA at the cellular membrane level of epithelial cells and monocyte-derived dendritic cells (MDDC), two potential target cells of HIV at the genital mucosal level.
Methods: The inhibitory effects of HHA and GNA were evaluated on HIV adsorption to genital epithelial HEC-1A cell line, on HIV transcytosis throughout a monolayer of polarized epithelial HEC-1A cells, on HIV adsorption to MDDC and on transfer of HIV from MDDC to autologous T lymphocytes.
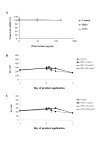
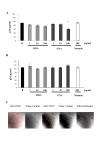
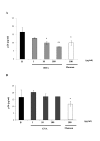
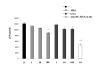
Results: HHA faintly inhibited attachment to HEC-1A cells of the R5-tropic HIV-1Ba-L strain, in a dose-dependent manner, whereas GNA moderately inhibited HIV adsorption in the same context, but only at high drug doses. Only HHA, but not GNA, inhibited HIV-1JR-CSF transcytosis in a dose-dependent manner. By confocal microscopy, HHA, but not GNA, was adsorbed at the epithelial cell surface, suggesting that HHA interacts specifically with receptors mediating HIV-1 transcytosis. Both plant lectins partially inhibited HIV attachment to MDDC. HHA inhibited more efficiently the transfer of HIV from MDDC to T cell, than GNA. Both HHA and GNA lacked toxicity below 200 microg/ml irrespective the cellular system used and do not disturb the monolayer integrity of epithelial cells.
Conclusion: These observations demonstrate higher inhibitory activities of the lectin plant HHA by comparison to GNA, on HIV adsorption to HEC-1A cell line, HIV transcytosis through HEC-1A cell line monolayer, HIV adsorption to MDDC and HIV transfer from MDDC to T cells, highlighting the potential interest of HHA as effective microbicide against HIV.
Figures

References
-
- Van Damme EJM., A. K. Allen, and W. J. Peumans. Related mannose-specific lectins from different species of the family Amaryllidaceae. Plant Physiol. 1988;73:52-57.
-
- Balzarini J, Hatse S, Vermeire K, Princen K, Aquaro S, Perno CF, De Clercq E, Egberink H, Vanden Mooter G, Peumans W, Van Damme E, Schols D. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother. 2004;48:3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. - DOI - PMC - PubMed
-
- Balzarini J, Van Herrewege Y, Vermeire K, Vanham G, Schols D. Carbohydrate-binding agents efficiently prevent dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-directed HIV-1 transmission to T lymphocytes. Mol Pharmacol. 2007;71:3–11. doi: 10.1124/mol.106.030155. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

