The control of viral infection by tripartite motif proteins and cyclophilin A
- PMID: 17565686
- PMCID: PMC1906832
- DOI: 10.1186/1742-4690-4-40
The control of viral infection by tripartite motif proteins and cyclophilin A
Abstract
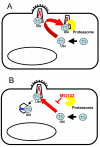
The control of retroviral infection by antiviral factors referred to as restriction factors has become an exciting area in infectious disease research. TRIM5alpha has emerged as an important restriction factor impacting on retroviral replication including HIV-1 replication in primates. TRIM5alpha has a tripartite motif comprising RING, B-Box and coiled coil domains. The antiviral alpha splice variant additionally encodes a B30.2 domain which is recruited to incoming viral cores and determines antiviral specificity. TRIM5 is ubiquitinylated and rapidly turned over by the proteasome in a RING dependent way. Protecting restricted virus from degradation, by inhibiting the proteasome, rescues DNA synthesis, but not infectivity, indicating that restriction of infectivity by TRIM5alpha does not depend on the proteasome but the early block to DNA synthesis is likely to be mediated by rapid degradation of the restricted cores. The peptidyl prolyl isomerase enzyme cyclophilin A isomerises a peptide bond on the surface of the HIV-1 capsid and impacts on sensitivity to restriction by TRIM5alpha from Old World monkeys. This suggests that TRIM5alpha from Old World monkeys might have a preference for a particular capsid isomer and suggests a role for cyclophilin A in innate immunity in general. Whether there are more human antiviral TRIMs remains uncertain although the evidence for TRIM19's (PML) antiviral properties continues to grow. A TRIM5-like molecule with broad antiviral activity in cattle suggests that TRIM mediated innate immunity might be common in mammals. Certainly the continued study of restriction of viral infectivity by antiviral host factors will remain of interest to a broad audience and impact on a variety of areas including development of animal models for infection, development of viral vectors for gene therapy and the search for novel antiviral drug targets.
Figures


References
-
- Shibata R, Sakai H, Kawamura M, Tokunaga K, Adachi A. Early replication block of human immunodeficiency virus type 1 in monkey cells. J Gen Virol. 1995;76:2723–2730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

