Reversine increases the plasticity of lineage-committed mammalian cells
- PMID: 17566101
- PMCID: PMC1965539
- DOI: 10.1073/pnas.0704360104
Reversine increases the plasticity of lineage-committed mammalian cells
Abstract
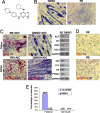
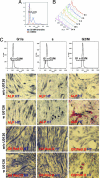
Previously, a small molecule, reversine, was identified that reverses lineage-committed murine myoblasts to a more primitive multipotent state. Here, we show that reversine can increase the plasticity of C2C12 myoblasts at the single-cell level and that reversine-treated cells gain the ability to differentiate into osteoblasts and adipocytes under lineage-specific inducing conditions. Moreover, reversine is active in multiple cell types, including 3T3E1 osteoblasts and human primary skeletal myoblasts. Biochemical and cellular experiments suggest that reversine functions as a dual inhibitor of nonmuscle myosin II heavy chain and MEK1, and that both activities are required for reversine's effect. Inhibition of MEK1 and nonmuscle myosin II heavy chain results in altered cell cycle and changes in histone acetylation status, but other factors also may contribute to the activity of reversine, including activation of the PI3K signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hochedlinger K, Jaenisch R. Nature. 2006;441:1061–1067. - PubMed
-
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Nature. 1997;385:810–813. - PubMed
-
- Cowan CA, Atienza J, Melton DA, Eggan K. Science. 2005;309:1369–1373. - PubMed
-
- Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Curr Biol. 2001;11:1553–1558. - PubMed
-
- Takahashi K, Yamanaka S. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

