Enhanced antibody production in mice to the malaria antigen AMA1 by CPG 7909 requires physical association of CpG and antigen
- PMID: 17566616
- PMCID: PMC1997297
- DOI: 10.1016/j.vaccine.2007.05.007
Enhanced antibody production in mice to the malaria antigen AMA1 by CPG 7909 requires physical association of CpG and antigen
Abstract
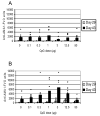
CpG oligodeoxynucleotides are potent immunostimulants. In this study, CPG 7909 was formulated with the recombinant Plasmodium falciparum protein AMA1-C1 adsorbed to Alhydrogel (aluminum hydroxide) and used to immunize mice. Mice receiving free CPG 7909 in a separate same site injection to the AMA1-C1/Alhydrogel had the same antibody responses as mice receiving AMA1-C1/Alhydrogel alone. For mice immunized with CPG 7909 bound to the AMA1-C1/Alhydrogel formulation, there was a bell shaped CPG 7909 dose-response curve with the highest antibody response co-incident with the concentration of CPG 7909 that saturated binding to the Alhydrogel. At a higher CPG 7909 dose where 74% was unbound, there was no enhancement of response over AMA1-C1/Alhydrogel alone. Our results suggest that the adjuvant effects of CpGs are optimal when adsorbed to Alhydrogel and highlight the need for careful characterization of the vaccine formulation.
Figures
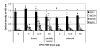
References
-
- Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol Rev. 2004;199:201–16. - PubMed
-
- Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374(6522):546–9. - PubMed
-
- Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. - PubMed
-
- Tighe H, Takabayashi K, Schwartz D, et al. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30(7):1939–47. - PubMed
-
- Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol. 2000;164(3):1617–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

