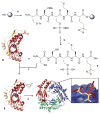
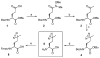
Covalent capture of phospho-dependent protein oligomerization by site-specific incorporation of a diazirine photo-cross-linker
- PMID: 17567014
- PMCID: PMC3242410
- DOI: 10.1021/ja072013j
Covalent capture of phospho-dependent protein oligomerization by site-specific incorporation of a diazirine photo-cross-linker
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

