Simultaneously physically and chemically gelling polymer system utilizing a poly(NIPAAm-co-cysteamine)-based copolymer
- PMID: 17567067
- PMCID: PMC2892919
- DOI: 10.1021/bm070267r
Simultaneously physically and chemically gelling polymer system utilizing a poly(NIPAAm-co-cysteamine)-based copolymer
Abstract
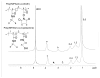
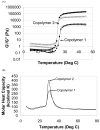
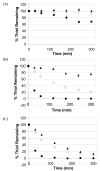
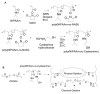
The objective of this work was to create an in situ physically and chemically cross-linking hydrogel for in vivo applications. N-Isopropylacrylamide (NIPAAm) was copolymerized with N-acryloxysuccinimide (NASI) via free radical polymerization. Poly(NIPAAm-co-NASI) was further modified to obtain poly(NIPAAm-co-cysteamine) through a nucleophilic attack on the carbonyl group of the NASI by the amine group of the cysteamine. Modification was verified by nuclear magnetic resonance. In addition to thermoresponsive physical gelling due to the presence of NIPAAm, this system also chemically gels via a Michael-type addition reaction when mixed with poly(ethylene glycol) diacrylate. The presence of both physical and chemical gelation resulted in material properties that are much improved compared to purely physical gels. The chemical gelation time of the copolymers was not significantly affected by the amount of thiol present due to the increased pKa of the copolymer containing more thiols. In addition, the swelling of the copolymers was highly dependent on the temperature and thiol content. Last, the rate of nucleophilic attack in the Michael-type addition reaction was shown to be highly dependent on pH and on the mole ratio of thiol to acrylate. Due to the improved mechanical properties, this material may be better suited for long-term functional replacement applications than other thermosensitive physical gels. With further development and biocompatibility testing, this material could potentially be applied as a temperature-responsive injectable biomaterial for functional embolization.
Figures




References
-
- Vernon BL, Tirelli N, Bachi T, Haldimann D, Hubbell JA. Water-borne, in situ crosslinked biomaterials from phase-segregated precursors. Journal of Biomedical Materials Research. 2002;64:447–456. - PubMed
-
- McLemore R, Preul MC, Vernon BL. Controlling Delivery Properties of a Waterborne, In-Situ-Forming Material. Journal of Biomedical Materials Research B. 2006 - PubMed
-
- Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2003;25:1339–1348. - PubMed
-
- Zhao C, Wang Q, Meng J, Liu W, Liu Z. A new thermosensitive polymer as noadhesive liquid embolism material. Current Applied Physics. 2005;5:497–500.
-
- Becker T, Kipke D, Preul M, Bichard W, McDougall C. In vivo assessment of calcium alginate gel for endovascular embolization of a cerebral arteriovenous malformation model using the Swine rete mirabile. Neurosurgery. 2002;51:453–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

