Finding intermediates in the O2 activation pathways of non-heme iron oxygenases
- PMID: 17567087
- PMCID: PMC2720168
- DOI: 10.1021/ar700052v
Finding intermediates in the O2 activation pathways of non-heme iron oxygenases
Abstract
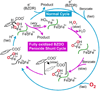
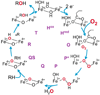
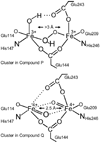
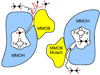
Intermediates in the reaction cycle of an oxygenase are usually very informative with respect to the chemical mechanism of O 2 activation and insertion. However, detection of these intermediates is often complicated by their short lifetime and the regulatory mechanism of the enzyme designed to ensure specificity. Here, the methods used to detect the intermediates in an extradiol dioxygenase, a Rieske cis-dihydrodiol dioxygenase, and soluble methane monooxygenase are discussed. The methods include the use of alternative, chromophoric substrates, mutagenesis of active site catalytic residues, forced changes in substrate binding order, control of reaction rates using regulatory proteins, and initialization of catalysis in crystallo.
Figures







References
-
- Dagley S. Biochemistry of Aromatic Hydrocarbon Degradation in Pseudomonads. In: Sokatch JR, editor. The Bacteria. Vol. 10. New York, N.Y: Academic Press; 1986. pp. 527–556.
-
- Lipscomb JD, Orville AM. Mechanistic Aspects of Dihydroxybenzoate Dioxygenases. In: Sigel H, Sigel I, editors. Metal Ions in Biological Systems. Vol. 28. New York: Marcel Dekker; 1992. pp. 243–298.
-
- Han S, Eltis LD, Timmis KN, Muchmore SW, Bolin JT. Crystal Structure of the Biphenyl-Cleaving Extradiol Dioxygenase from a PCB-Degrading Pseudomonad. Science. 1995;270:976–980. - PubMed
-
- Senda T, Sugiyama K, Narita H, Yamamoto T, Kimbara K, Fukuda M, Sato M, Yano K, Mitsui Y. Three-Dimensional Structures of Free Form and Two Substrate Complexes of an Extradiol Ring-Cleavage Type Dioxygenase the BphC Enzyme from Pseudomonas sp. Strain KKS102. J. Mol. Biol. 1996;255:735–752. - PubMed
-
- Hegg EL, Que L., Jr The 2-His-1-Carboxylate Facial Triad. An Emerging Structural Motif in Mononuclear Non-Heme Iron(II) Eur Enzymes. J. Biochem. 1997;250:625–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

