Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms
- PMID: 17567242
- PMCID: PMC2443402
- DOI: 10.1089/ars.2007.1674
Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms
Abstract
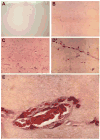
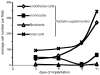
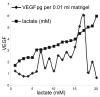
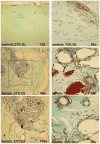
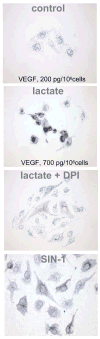
Hypoxia serves as a physiologic cue to drive an angiogenic response via HIF-dependent mechanisms. Interestingly, minor elevation of lactate levels in the tissue produces the same effect under aerobic conditions. Aerobic glycolysis contributes to lactate accumulation in the presence of oxygen, especially under inflammatory conditions. We previously postulated that aerobic lactate accumulation, already known to stimulate collagen deposition, will also stimulate angiogenesis. If substantiated, this concept would advance understanding of wound healing and aerobic angiogenesis because lactate accumulation has many aerobic sources. In this study, Matrigel plugs containing a powdered, hydrolyzable lactate polymer were implanted into the subcutaneous space of mice. Lactate monomer concentrations in the implant were consistent with wound levels for more than 11 days. They induced little inflammation but considerable VEGF production and were highly angiogenic, as opposed to controls. Arterial hypoxia abrogated angiogenesis. Furthermore, inhibition of lactate dehydrogenase by using oxamate also prevented the angiogenic effects of lactate. Lactate monomer, at concentrations found in cutaneous wounds, stabilized HIF-1alpha and increased VEGF levels in aerobically cultured human endothelial cells. Accumulated lactate, therefore, appears to convey the impression of "metabolic need" for vascularization, even in well-oxygenated and pH-neutral conditions. Lactate and oxygen together stimulate angiogenesis and matrix deposition.
Figures



References
-
- Albina JE, Mastrofrancesco B, Vessella JA, Louis CA, Henry WL, Jr, Reichner JS. HIF-1 expression in healing wounds: HIF-1alpha induction in primary inflammatory cells by TNF-alpha. Am J Physiol Cell Physiol. 2001;281:C1971–1977. - PubMed
-
- Ali MA, Yasui F, Matsugo S, Konishi T. The lactate-dependent enhancement of hydroxyl radical generation by the Fenton reaction. Free Radic Res. 2000;32:429–438. - PubMed
-
- Allen DB, Maguire JJ, Mahdavian M, Wicke C, Marcocci L, Scheuenstuhl H, Chang M, Le AX, Hopf HW, Hunt TK. Wound hypoxia and acidosis limit neutrophil bacterial killing mechanisms. Arch Surg. 1997;132:991–996. - PubMed
-
- Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48:1460–1470. - PubMed
-
- Beckert S, Farrahi F, Aslam RS, Scheuenstuhl H, Konigsrainer A, Hussain MZ, Hunt TK. Lactate stimulates endothelial cell migration. Wound Repair Regen. 2006;14:321–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

