Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(-/-)/Aldh1a1(-/-) knock-out mice
- PMID: 17567582
- PMCID: PMC2253645
- DOI: 10.1074/jbc.M702076200
Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(-/-)/Aldh1a1(-/-) knock-out mice
Abstract
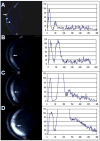
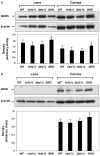
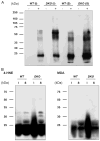
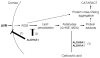
ALDH3A1 (aldehyde dehydrogenase 3A1) is abundant in the mouse cornea but undetectable in the lens, and ALDH1A1 is present at lower (catalytic) levels in the cornea and lens. To test the hypothesis that ALDH3A1 and ALDH1A1 protect the anterior segment of the eye against environmentally induced oxidative damage, Aldh1a1(-/-)/Aldh3a1(-/-) double knock-out and Aldh1a1(-/-) and Aldh3a1(-/-) single knock-out mice were evaluated for biochemical changes and cataract formation (lens opacification). The Aldh1a1/Aldh3a1- and Aldh3a1-null mice develop cataracts in the anterior and posterior subcapsular regions as well as punctate opacities in the cortex by 1 month of age. The Aldh1a1-null mice also develop cataracts later in life (6-9 months of age). One- to three-month-old Aldh-null mice exposed to UVB exhibited accelerated anterior lens subcapsular opacification, which was more pronounced in Aldh3a1(-/-) and Aldh3a1(-/-)/Aldh1a1(-/-) mice compared with Aldh1a1(-/-) and wild type animals. Cataract formation was associated with decreased proteasomal activity, increased protein oxidation, increased GSH levels, and increased levels of 4-hydroxy-2-nonenal- and malondialdehyde-protein adducts. In conclusion, these findings support the hypothesis that corneal ALDH3A1 and lens ALDH1A1 protect the eye against cataract formation via nonenzymatic (light filtering) and enzymatic (detoxification) functions.
Figures

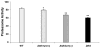
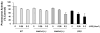
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

