Chemical cross-linking of the chloroplast localized small heat-shock protein, Hsp21, and the model substrate citrate synthase
- PMID: 17567739
- PMCID: PMC2206695
- DOI: 10.1110/ps.072831607
Chemical cross-linking of the chloroplast localized small heat-shock protein, Hsp21, and the model substrate citrate synthase
Abstract
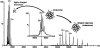
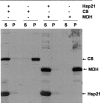
The molecular mechanism whereby the small heat-shock protein (sHsp) chaperones interact with and prevent aggregation of other proteins is not fully understood. We have characterized the sHsp-substrate protein interaction at normal and increased temperatures utilizing a model substrate protein, citrate synthase (CS), widely used in chaperone assays, and a dodecameric plant sHsp, Hsp21, by chemical cross-linking with 3,3'-Dithiobis[sulfosuccinimidylpropionate] (DTSSP) and mass spectrometric peptide mapping. In the absence of CS, the cross-linker captured Hsp21 in dodecameric form, even at increased temperature (47 degrees C). In the presence of equimolar amounts of CS, no Hsp21 dodecamer was captured, indicating a substrate-induced Hsp21 dodecamer dissociation by equimolar amounts of CS. Cross-linked Hsp21-Hsp21 dipeptides indicated an exposure of the Hsp21 C-terminal tails and substrate-binding sites normally covered by the C terminus. Cross-linked Hsp21-CS dipeptides mapped to several sites on the surface of the CS dimer, indicating that there are numerous weak and short-lived interactions between Hsp21 and CS, even at normal temperatures. The N-terminal arms especially interacted with a motif in the CS dimer, which is absent in thermostable forms of CS. The cross-linking data suggest that the presence of substrate rather than temperature influences the conformation of Hsp21.
Figures






References
-
- Åhrman E., Gustavsson, N., Hultschig, C., Boelens, W., and Sundby Emanuelsson, C. E. 2007. Small heat shock proteins prevent aggregation of citrate synthase and bind to the N-terminal region which is absent in thermostable forms of citrate synthase. Extremophiles (in press). - PubMed
-
- Aquilina J.A. and Watt, S.J. 2007. The N-terminal domain of αB-crystallin is protected from proteolysis by bound substrate. Biochem. Biophys. Res. Commun. 353: 1115–1120. - PubMed
-
- Arnott M.A., Michael, R.A., Thompson, C.R., Hough, D.W., and Danson, M.J. 2000. Thermostability and thermoactivity of citrate synthases from the thermophilic and hyperthermophilic archaea, Thermoplasma acidophilum and Pyrococcus furiosus . J. Mol. Biol. 304: 657–668. - PubMed
-
- Basha E., Lee, G.J., Breci, L.A., Hausrath, A.C., Buan, N.R., Giese, K.C., and Vierling, E. 2004. The identity of proteins associated with a small heat shock protein during heat stress in vivo indicates that these chaperones protect a wide range of cellular functions. J. Biol. Chem. 279: 7566–7575. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

