A high-throughput method for membrane protein solubility screening: the ultracentrifugation dispersity sedimentation assay
- PMID: 17567744
- PMCID: PMC2206705
- DOI: 10.1110/ps.072759907
A high-throughput method for membrane protein solubility screening: the ultracentrifugation dispersity sedimentation assay
Erratum in
- Protein Sci. 2007 Dec;16(12):2775. Postis, Vincent [added]; Xia, Xiaobing [added]
Abstract
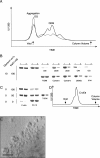
One key to successful crystallization of membrane proteins is the identification of detergents that maintain the protein in a soluble, monodispersed state. Because of their hydrophobic nature, membrane proteins are particularly prone to forming insoluble aggregates over time. This nonspecific aggregation of the molecules reduces the likelihood of the regular association of the protein molecules essential for crystal lattice formation. Critical buffer components affecting the aggregation of membrane proteins include detergent choice, salt concentration, and presence of glycerol. The optimization of these parameters is often a time- and protein-consuming process. Here we describe a novel ultracentrifugation dispersity sedimentation (UDS) assay in which ultracentrifugation of very small (5 microL) volumes of purified, soluble membrane protein is combined with SDS-PAGE analysis to rapidly assess the degree of protein aggregation. The results from the UDS method correlate very well with established methods like size-exclusion chromatography (SEC), while consuming considerably less protein. In addition, the UDS method allows rapid screening of detergents for membrane protein crystallization in a fraction of the time required by SEC. Here we use the UDS method in the identification of suitable detergents and buffer compositions for the crystallization of three recombinant prokaryotic membrane proteins. The implications of our results for membrane protein crystallization prescreening are discussed.
Figures
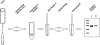


References
-
- Byrne B. and Jormakka, M. 2006. Solubilization and purification of membrane proteins. In Structural genomics on membrane proteins (ed. K.H. Lundstrom), pp. 179–198. Taylor and Francis, New York.
-
- Chang G., Spencer, R.H., Lee, A.T., Barclay, M.T., and Rees, D.C. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: A gated mechanosensitive ion channel. Science 282: 2220–2226. - PubMed
-
- D'Arcy A. 1994. Crystallizing proteins—A rational approach? Acta Crystallogr. D Biol. Crystallogr. 50: 469–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

