IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development
- PMID: 17567755
- PMCID: PMC1965558
- DOI: 10.1073/pnas.0702072104
IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development
Abstract
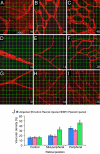
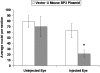
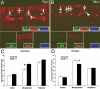
We asked whether the hypoxia-regulated factor, insulin-like growth factor binding protein-3 (IGFBP3), could modulate stem cell factor receptor (c-kit+), stem cell antigen-1 (sca-1+), hematopoietic stem cell (HSC), or CD34+ endothelial precursor cell (EPC) function. Exposure of CD34+ EPCs to IGFBP3 resulted in rapid differentiation into endothelial cells and dose-dependent increases in cell migration and capillary tube formation. IGFBP3-expressing plasmid was injected into the vitreous of neonatal mice undergoing the oxygen-induced retinopathy (OIR) model. In separate studies, GFP-expressing HSCs were transfected with IGFBP3 plasmid and injected into the vitreous of OIR mice. Administering either IGFBP3 plasmid alone or HSCs transfected with the plasmid resulted in a similar reduction in areas of vasoobliteration, protection of the developing vasculature from hyperoxia-induced regression, and reduction in preretinal neovascularization compared to control plasmid or HSCs transfected with control plasmid. In conclusion, IGFBP3 mediates EPC migration, differentiation, and capillary formation in vitro. Targeted expression of IGFBP3 protects the vasculature from damage and promotes proper vascular repair after hyperoxic insult in the OIR model. IGFBP3 expression may represent a physiological adaptation to ischemia and potentially a therapeutic target for treatment of ischemic conditions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, Robinson G, Driver S, Bischoff J, Zhang B, et al. Nat Med. 1999;5:1390–1395. - PubMed
-
- Hellstrom A, Engstrom E, Hard AL, Albertsson-Wikland K, Carlsson B, Niklasson A, Lofqvist C, Svensson E, Holm S, Ewald U, et al. Pediatrics. 2003;112:1016–1020. - PubMed
-
- Lofqvist C, Engstrom E, Sigurdsson J, Hard AL, Niklasson A, Ewald U, Holmstrom G, Smith LE, Hellstrom A. Pediatrics. 2006;117:1930–1938. - PubMed
-
- Grant MB, Russell B, Fitzgerald C, Merimee TJ. Diabetes. 1986;35:416–420. - PubMed
-
- Grant MB, Wargovich TJ, Ellis EA, Tarnuzzer R, Caballero S, Estes K, Rossing M, Spoerri PE, Pepine C. Regul Pept. 1996;67:137–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

