IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth
- PMID: 17567756
- PMCID: PMC1965557
- DOI: 10.1073/pnas.0702031104
IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth
Abstract
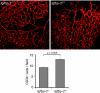
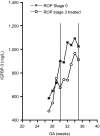
Vessel loss precipitates many diseases. In particular, vessel loss resulting in hypoxia induces retinal neovascularization in diabetic retinopathy and in retinopathy of prematurity (ROP), major causes of blindness. Here we define insulin-like growth factor binding protein-3 (IGFBP3) as a new modulator of vascular survival and regrowth in oxygen-induced retinopathy. In IGFBP3-deficient mice, there was a dose-dependent increase in oxygen-induced retinal vessel loss. Subsequent to oxygen-induced retinal vessel loss, Igfbp3(-/-) mice had a 31% decrease in retinal vessel regrowth versus controls after returning to room air. No difference in serum insulin-like growth factor 1 (IGF1) levels was observed among groups. Wild-type mice treated with exogenous IGFBP3 had a significant increase in vessel regrowth. This correlated with a 30% increase in endothelial progenitor cells in the retina at postnatal day 15, indicating that IGFBP3 could be serving as a progenitor cell chemoattractant. In a prospective clinical study, we measured IGFBP3 (and IGF1) plasma levels weekly and examined retinas in all premature infants born at gestational ages <32 weeks at high risk for ROP. The mean level of IGFBP3 at 30-35 weeks postmenstrual age (PMA) for infants with proliferative ROP (ROP stages 3>, n = 13) was 802 microg/liter, and for infants with no ROP (ROP stage 0, n = 38) the mean level was 974 microg/liter (P < 0.03). These results suggest that IGFBP3, acting independently of IGF1, helps to prevent oxygen-induced vessel loss and to promote vascular regrowth after vascular destruction in vivo in a dose-dependent manner, resulting in less retinal neovascularization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hellstrom A, Engstrom E, Hard AL, Albertsson-Wikland K, Carlsson B, Niklasson A, Lofqvist C, Svensson E, Holm S, Ewald U, et al. Pediatrics. 2003;112:1016–1020. - PubMed
-
- Mohan S, Baylink DJ. J Endocrinol. 2002;175:19–31. - PubMed
-
- Firth SM, Baxter RC. Endocr Rev. 2002;23:824–854. - PubMed
-
- De Mellow JS, Baxter RC. Biochem Biophys Res Commun. 1988;156:199–204. - PubMed
-
- Conover CA. Endocrinology. 1992;130:3191–3319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

