Differential role of N-type calcium channel splice isoforms in pain
- PMID: 17567797
- PMCID: PMC6672448
- DOI: 10.1523/JNEUROSCI.0307-07.2007
Differential role of N-type calcium channel splice isoforms in pain
Abstract
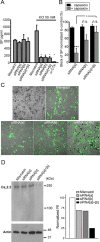
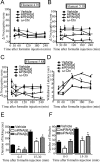
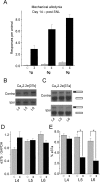
N-type calcium channels are essential mediators of spinal nociceptive transmission. The core subunit of the N-type channel is encoded by a single gene, and multiple N-type channel isoforms can be generated by alternate splicing. In particular, cell-specific inclusion of an alternatively spliced exon 37a generates a novel form of the N-type channel that is highly enriched in nociceptive neurons and, as we show here, downregulated in a neuropathic pain model. Splice isoform-specific small interfering RNA silencing in vivo reveals that channels containing exon 37a are specifically required for mediating basal thermal nociception and for developing thermal and mechanical hyperalgesia during inflammatory and neuropathic pain. In contrast, both N-type channel isoforms (e37a- and e37b-containing) contribute to tactile neuropathic allodynia. Hence, exon 37a acts as a molecular switch that tailors the channels toward specific roles in pain.
Figures



References
-
- Altier C, Zamponi GW. Targeting Ca2+ channels to treat pain: T-type versus N-type. Trends Pharmacol Sci. 2004;25:465–470. - PubMed
-
- Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, Dubel SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. - PubMed
-
- Beedle AM, McRory JE, Poirot O, Doering CJ, Altier C, Barrere C, Hamid J, Nargeot J, Bourinet E, Zamponi GW. Agonist-independent modulation of N-type calcium channels by ORL1 receptors. Nat Neurosci. 2004;7:118–125. - PubMed
-
- Bell TJ, Thaler C, Castiglioni AJ, Helton TD, Lipscombe D. Cell-specific alternative splicing increases calcium channel current density in the pain pathway. Neuron. 2004;41:127–138. - PubMed
-
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
