Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning
- PMID: 17567809
- PMCID: PMC6672440
- DOI: 10.1523/JNEUROSCI.0342-07.2007
Molecular characterization of the ankle-link complex in cochlear hair cells and its role in the hair bundle functioning
Abstract
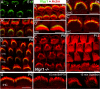
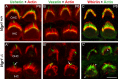
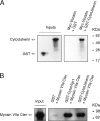
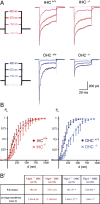
Several lines of evidence indicate that very large G-protein-coupled receptor 1 (Vlgr1) makes up the ankle links that connect the stereocilia of hair cells at their base. Here, we show that the transmembrane protein usherin, the putative transmembrane protein vezatin, and the PDZ (postsynaptic density-95/Discs large/zona occludens-1) domain-containing submembrane protein whirlin are colocalized with Vlgr1 at the stereocilia base in developing cochlear hair cells and are absent in Vlgr1-/- mice that lack the ankle links. Direct in vitro interactions between these four proteins further support their involvement in a molecular complex associated with the ankle links and scaffolded by whirlin. In addition, the delocalization of these proteins in myosin VIIa defective mutant mice as well as the myosin VIIa tail direct interactions with vezatin, whirlin, and, we show, Vlgr1 and usherin, suggest that myosin VIIa conveys proteins of the ankle-link complex to the stereocilia. Adenylyl cyclase 6, which was found at the base of stereocilia, was both overexpressed and mislocated in Vlgr1-/- mice. In postnatal day 7 Vlgr1-/- mice, mechanoelectrical transduction currents evoked by displacements of the hair bundle toward the tallest stereocilia (i.e., in the excitatory direction) were reduced in outer but not inner hair cells. In both cell types, stimulation of the hair bundle in the opposite direction paradoxically resulted in significant transduction currents. The absence of ankle-link-mediated cohesive forces within hair bundles lacking Vlgr1 may account for the electrophysiological results. However, because some long cadherin-23 isoforms could no longer be detected in Vlgr1-/- mice shortly after birth, the loss of some apical links could be involved too. The premature disappearance of these cadherin isoforms in the Vlgr1-/- mutant argues in favor of a signaling function of the ankle links in hair bundle differentiation.
Figures





References
-
- Adato A, Kikkawa Y, Reiners J, Alagramam KN, Weil D, Yonekawa H, Wolfrum U, El-Amraoui A, Petit C. Interactions in the network of Usher syndrome type 1 proteins. Hum Mol Genet. 2005a;14:347–356. - PubMed
-
- Adato A, Lefèvre G, Delprat B, Michel V, Michalski N, Chardenoux S, Weil D, El-Amraoui A, Petit C. Usherin, the defective protein in Usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet. 2005b;14:3921–3932. - PubMed
-
- Alagramam KN, Yuan H, Kuehn MH, Murcia CL, Wayne S, Srisailpathy CRS, Lowry RB, Knaus R, Van Laer L, Bernier FP, Schwartz S, Lee C, Morton CC, Mullins RF, Ramesh A, Van Camp G, Hagemen GS, Woychik RP, Smith RJH. Mutations in the novel protocadherin PCDH15 cause Usher syndrome type 1F. Hum Mol Genet. 2001;10:1709–1718. - PubMed
-
- Appert-Collin A, Baisamy L, Diviani D. Regulation of G protein-coupled receptor signaling by A-kinase anchoring proteins. J Recept Signal Transduct Res. 2006;26:631–646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
