Resolvin E1 and protectin D1 activate inflammation-resolution programmes
- PMID: 17568749
- PMCID: PMC2757086
- DOI: 10.1038/nature05877
Resolvin E1 and protectin D1 activate inflammation-resolution programmes
Abstract
Resolution of acute inflammation is an active process essential for appropriate host responses, tissue protection and the return to homeostasis. During resolution, specific omega-3 polyunsaturated fatty-acid-derived mediators are generated within resolving exudates, including resolvin E1 (RvE1) and protectin D1 (PD1). It is thus important to pinpoint specific actions of RvE1 and PD1 in regulating tissue resolution. Here we report that RvE1 and PD1 in nanogram quantities promote phagocyte removal during acute inflammation by regulating leukocyte infiltration, increasing macrophage ingestion of apoptotic polymorphonuclear neutrophils in vivo and in vitro, and enhancing the appearance of phagocytes carrying engulfed zymosan in lymph nodes and spleen. In this tissue terrain, inhibition of either cyclooxygenase or lipoxygenases--pivotal enzymes in the temporal generation of both pro-inflammatory and pro-resolving mediators--caused a 'resolution deficit' that was rescued by RvE1, PD1 or aspirin-triggered lipoxin A4 analogue. Also, new resolution routes were identified that involve phagocytes traversing perinodal adipose tissues and non-apoptotic polymorphonuclear neutrophils carrying engulfed zymosan to lymph nodes. Together, these results identify new active components for postexudate resolution traffic, and demonstrate that RvE1 and PD1 are potent agonists for resolution of inflamed tissues.
Figures
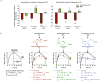
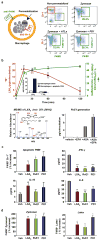
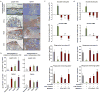
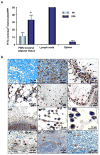
References
-
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. - PubMed
-
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. - PubMed
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. - PubMed
-
- Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

