An in vitro model system to study gene therapy in the human inner ear
- PMID: 17568767
- PMCID: PMC2742230
- DOI: 10.1038/sj.gt.3302980
An in vitro model system to study gene therapy in the human inner ear
Abstract
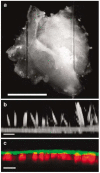
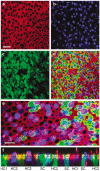
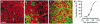
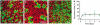
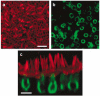
The confined fluid-filled labyrinth of the human inner ear presents an opportunity for introduction of gene therapy reagents designed to treat hearing and balance dysfunction. Here we present a novel model system derived from the sensory epithelia of human vestibular organs and show that the tissue can survive up to 5 days in vitro. We generated organotypic cultures from 26 human sensory epithelia excised at the time of labyrinthectomy for intractable Meniere's disease or vestibular schwannoma. We applied multiply deleted adenoviral vectors at titers between 10(5) and 10(8) viral particles/ml directly to the cultures for 4-24 h and examined the tissue 12-96 h post-transfection. We noted robust expression of the exogenous transgene, green fluorescent protein (GFP), in hair cells and supporting cells suggesting both were targets of adenoviral transfection. We also transfected cultures with a vector that carried the genes for GFP and KCNQ4, a potassium channel subunit that causes dominant-progressive hearing loss when mutated. We noted a positive correlation between GFP fluorescence and KCNQ4 immunolocalization. We conclude that our in vitro model system presents a novel and effective experimental paradigm for evaluation of gene therapy reagents designed to restore cellular function in patients who suffer from inner ear disorders.
Figures


References
-
- Petit C. From deafness genes to hearing mechanisms: harmony and counterpoint. Trends Mol Med. 2006;12:57–64. - PubMed
-
- Holt JR. Viral-mediated gene transfer to study the molecular physiology of the mammalian inner ear. Audiol Neurootol. 2002;7:157–160. - PubMed
-
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. - PubMed
-
- Luebke AE, Steiger JD, Hodges BL, Amalfitano A. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 2001;8:789–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

