Evidence for a dynamic and transient pathway through the TAT protein transport machinery
- PMID: 17568769
- PMCID: PMC1914107
- DOI: 10.1038/sj.emboj.7601759
Evidence for a dynamic and transient pathway through the TAT protein transport machinery
Abstract
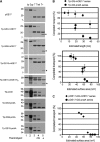
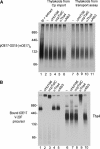
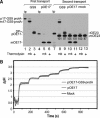
Tat systems transport completely folded proteins across ion-tight membranes. Three membrane proteins comprise the Tat machinery in most systems. In thylakoids, cpTatC and Hcf106 mediate precursor recognition, whereas Tha4 facilitates translocation. We used chimeric precursor proteins with unstructured peptides and folded domains to test predictions of competing translocation models. Two models invoke protein-conducting channels, whereas another model proposes that cpTatC pulls substrates through a patch of Tha4 on the lipid bilayer. The thylakoid system transported unstructured peptide substrates alone or when fused to folded domains. However, larger substrates stalled before completion, some with amino- and carboxyl-folded domains on opposite sides of the membrane. The length of the precursor that resulted in translocation arrest (20 to 30 nm) exceeded that expected for a single 'pull' mechanism, suggesting that a sustained driving force rather than a single pull moves the protein across the bilayer. Three different methods showed that stalled substrates were not stuck in a channel or even associated with Tat machinery. This finding favors the Tha4 patch model for translocation.
Figures






References
-
- Alami M, Luke I, Deitermann S, Eisner G, Koch HG, Brunner J, Muller M (2003) Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol Cell 12: 937–946 - PubMed
-
- Alder NN, Theg SM (2003) Energetics of protein transport across biological membranes. A study of the thylakoid DeltapH-dependent/cpTat pathway. Cell 112: 231–242 - PubMed
-
- Asai T, Shinoda Y, Nohara T, Yoshihisa T, Endo T (1999) Sec-dependent pathway and DeltapH-dependent pathway do not share a common translocation pore in thylakoidal protein transport. J Biol Chem 274: 20075–20078 - PubMed
-
- Balsera M, Arellano JB, Revuelta JL, de las Rivas J, Hermoso JA (2005) The 1.49 Å resolution crystal structure of PsbQ from photosystem II of Spinacia oleracea reveals a PPII structure in the N-terminal region. J Mol Biol 350: 1051–1060 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

