Protein kinase Cepsilon (PKCepsilon) and Src control PKCdelta activation loop phosphorylation in cardiomyocytes
- PMID: 17569658
- PMCID: PMC2901534
- DOI: 10.1074/jbc.M701676200
Protein kinase Cepsilon (PKCepsilon) and Src control PKCdelta activation loop phosphorylation in cardiomyocytes
Abstract
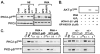
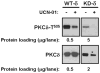
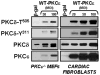
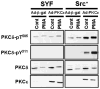
Protein kinase Cdelta (PKCdelta) is unusual among AGC kinases in that it does not require activation loop (Thr(505)) phosphorylation for catalytic competence. Nevertheless, Thr(505) phosphorylation has been implicated as a mechanism that influences PKCdelta activity. This study examines the controls of PKCdelta-Thr(505) phosphorylation in cardiomyocytes. We implicate phosphoinositide-dependent kinase-1 and PKCdelta autophosphorylation in the "priming" maturational PKCdelta-Thr(505) phosphorylation that accompanies de novo enzyme synthesis. In contrast, we show that PKCdelta-Thr(505) phosphorylation dynamically increases in cardiomyocytes treated with phorbol 12-myristate 13-acetate or the alpha(1)-adrenergic receptor agonist norepinephrine via a mechanism that requires novel PKC isoform activity and not phosphoinositide-dependent kinase-1. We used a PKCepsilon overexpression strategy as an initial approach to discriminate two possible novel PKC mechanisms, namely PKCdelta-Thr(505) autophosphorylation and PKCdelta-Thr(505) phosphorylation in trans by PKCepsilon. Our studies show that adenovirus-mediated PKCepsilon overexpression leads to an increase in PKCdelta-Thr(505) phosphorylation. However, this cannot be attributed to an effect of PKCepsilon to function as a direct PKCdelta-Thr(505) kinase, since the PKCepsilon-dependent increase in PKCdelta-Thr(505) phosphorylation is accompanied by (and dependent upon) increased PKCdelta phosphorylation at Tyr(311) and Tyr(332). Further studies implicate Src in this mechanism, showing that 1) PKCepsilon overexpression increases PKCdelta-Thr(505) phosphorylation in cardiomyocytes and Src(+) cells but not in SYF cells (that lack Src, Yes, and Fyn and exhibit a defect in PKCdelta-Tyr(311)/Tyr(332) phosphorylation), and 2) in vitro PKCdelta-Thr(505) autophosphorylation is augmented in assays performed with Src (which promotes PKCdelta-Tyr(311)/Tyr(332) phosphorylation). Collectively, these results identify a novel PKCdelta-Thr(505) autophosphorylation mechanism that is triggered by PKCepsilon overexpression and involves Src-dependent PKCdelta-Tyr(311)/Tyr(332) phosphorylation.
Figures




References
-
- Rybin VO, Sabri A, Short J, Braz JC, Molkentin JD, Steinberg SF. J Biol Chem. 2003;278:14555–14564. - PubMed
-
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Science. 1998;281:2042–2045. - PubMed
-
- Stempka L, Schnolzer M, Radke S, Rincke G, Marks F, Gschwendt M. J Biol Chem. 1999;274:8886–8892. - PubMed
-
- Liu Y, Belkina NV, Graham C, Shaw S. J Biol Chem. 2006;281:12102–12111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

