Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes
- PMID: 17569730
- PMCID: PMC2277233
- DOI: 10.1113/jphysiol.2007.136879
Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes
Abstract
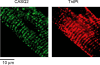
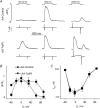
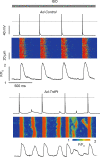
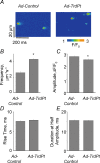
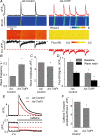
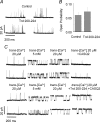
In cardiac muscle, intracellular Ca2+ release is controlled by a number of proteins including the ryanodine receptor (RyR2), calsequestrin (CASQ2), triadin-1 (Trd) and junctin (Jn) which form a complex in the junctional sarcoplasmic reticulum (SR) membrane. Within this complex, Trd appears to link CASQ2 to RyR2 although the functional significance of this interaction is unclear. In this study, we explored the functional importance of Trd-CASQ2 interactions for intracellular Ca2+ handling in rat ventricular myocytes. A peptide encompassing the homologous CASQ2 binding domain of Trd (residues 206-230 in the rat; TrdPt) was expressed in the lumen of the SR to disrupt Trd-CASQ2 interactions. Myocytes expressing TrdPt exhibited increased responsiveness of SR Ca2+ release to activation by ICa as manifested by flattened and broadened voltage dependency of the amplitude of cytosolic Ca2+ transients. Rhythmically paced, TrdPt-expressing myocytes exhibited spontaneous arrhythmogenic oscillations of intracellular Ca2+ and membrane potential that was not seen in control cells. In addition, the frequency of spontaneous Ca2+ sparks and Ca2+ waves was significantly increased in TrdPt-expressing, permeabilized myocytes. These alterations in SR Ca2+ release were accompanied by a significant decrease in basal free intra-SR[Ca2+] and total SR Ca2+ content in TrdPt-expressing cells. At the same time a synthetic peptide corresponding to the CASQ2 binding domain of Trd produced no direct effects on the activity of single RyR2 channels incorporated into lipid bilayers while interfering with the ability of CASQ2 to inhibit the RyR2 channel. These results suggest that CASQ2 stabilizes SR Ca2+ release by inhibiting the RyR2 channel through interaction with Trd. They also show that intracellular Ca2+ cycling in the heart relies on coordinated interactions between proteins of the RyR2 channel complex and that disruption of these interactions may represent a molecular mechanism for cardiac disease.
Figures
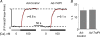
Comment in
-
An Il-lumen-ating look at ryanodine receptor modulation by disruption in triadin and calsequestrin interactions in cardiac myocytes.J Physiol. 2008 Feb 1;586(3):697-9. doi: 10.1113/jphysiol.2007.147751. Epub 2007 Nov 29. J Physiol. 2008. PMID: 18048447 Free PMC article. No abstract available.
References
-
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. - PubMed
-
- Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–429. - PubMed
-
- Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. - PubMed
-
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77:699–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

