Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle
- PMID: 17569733
- PMCID: PMC2277221
- DOI: 10.1113/jphysiol.2007.135046
Store-operated Ca2+ entry during intracellular Ca2+ release in mammalian skeletal muscle
Abstract
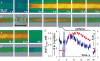
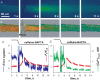
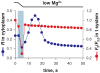
Store-operated Ca2+ entry (SOCE) is activated following the depletion of internal Ca2+ stores in virtually all eukaryotic cells. Shifted excitation and emission ratioing of fluorescence (SEER) was used to image mag-indo-1 trapped in the tubular (t) system of mechanically skinned rat skeletal muscle fibres to measure SOCE during intracellular Ca2+ release. Cytosolic Ca2+ transients were simultaneously imaged using the fluorescence of rhod-2. Spatially and temporally resolved images of t system [Ca2+] ([Ca2+]t-sys) allowed estimation of Ca2+ entry flux from the rate of decay of [Ca2+]t-sys. Ca2+ release was induced pharmacologically to activate SOCE without voltage-dependent contributions to Ca2+ flux. Inward Ca2+ flux was monotonically dependent on the [Ca2+] gradient, and strongly dependent on the transmembrane potential. The activation of SOCE was controlled locally. It could occur without full Ca2+ store depletion and in less than a second after initiation of store depletion. These results indicate that the molecular agonists of SOCE must be evenly distributed throughout the junctional membranes and can activate rapidly. Termination of SOCE required a net increase in [Ca2+]SR. Activation and termination of SOCE are also demonstrated, for the first time, during a single event of Ca2+ release. At the physiological [Ca2+]t-sys, near 2 mM (relative to t system volume), SOCE flux relative to accessible cytoplasmic volume was at least 18.6 microM s(-1), consistent with times of SR refilling of 1-2 min measured in intact muscle fibres.
Figures






Comment in
-
Store-operated Ca2+ release in skeletal muscle: tailored for a specialized system.J Physiol. 2007 Aug 15;583(Pt 1):1. doi: 10.1113/jphysiol.2007.139923. Epub 2007 Jul 12. J Physiol. 2007. PMID: 17627981 Free PMC article. No abstract available.
References
-
- Cheung A, Dantzig JA, Hollingworth S, Baylor SM, Goldman YE, Mitchison TJ, Straight AF. A small-molecule inhibitor of skeletal muscle myosin II. Nat Cell Biol. 2002;4:83–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

