Developmental changes in brain-derived neurotrophic factor-mediated modulations of synaptic activities in the pontine Kölliker-Fuse nucleus of the rat
- PMID: 17569735
- PMCID: PMC2277243
- DOI: 10.1113/jphysiol.2007.134726
Developmental changes in brain-derived neurotrophic factor-mediated modulations of synaptic activities in the pontine Kölliker-Fuse nucleus of the rat
Abstract
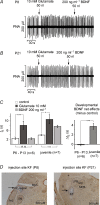
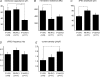
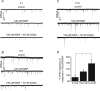
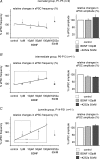
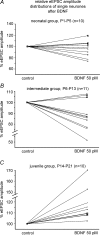
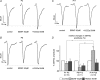
The Kölliker-Fuse nucleus (KF), part of the respiratory network, is involved in the modulation of respiratory phase durations in response to peripheral and central afferent inputs. The KF is immature at birth. Developmental changes in its physiological and anatomical properties have yet to be investigated. Since brain-derived neurotrophic factor (BDNF) is of major importance for the maturation of neuronal networks, we investigated its effects on developmental changes in the KF on different postnatal days (neonatal, P1-5; intermediate, P6-13; juvenile, P14-21) by analysing single neurones in the in vitro slice preparation and network activities in the perfused brainstem preparation in situ. The BDNF had only weak effects on the frequency of mixed excitatory and inhibitory spontaneous postsynaptic currents (sPSCs) in neonatal slice preparations. Postnatally, in the intermediate and juvenile age groups, a significant augmentation of the sPSC frequency was observed in the presence of 100 pm BDNF (+23.5+/-12.6 and +76.7+/-28.4%, respectively). Subsequent analyses of BDNF effects on evoked excitatory postsynaptic currents (eEPSCs) revealed significant enhancement of eEPSC amplitude of +20.8+/-7.0% only in juvenile stages (intermediates, -13.2+/-4.8%). On the network level, significant modulation of phrenic nerve activity following BDNF microinjection into the KF was also observed only in juveniles. The data suggest that KF neurones are subject to BDNF-mediated fast synaptic modulation after completion of postnatal maturation. After maturation, BDNF contributes to modulation of fast excitatory neurotransmission in respiratory-related KF neurones. This may be important for network plasticity associated with the processing of afferent information.
Figures

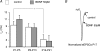
References
-
- Alheid GF, Milsom WK, McCrimmon DR. Pontine influences on breathing: an overview. Respir Physiol Neurobiol. 2004;143:105–114. - PubMed
-
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. - PubMed
-
- Arvanian VL, Mendell LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur J Neurosci. 2001a;14:1800–1808. - PubMed
-
- Arvanian VL, Mendell LM. Removal of NMDA receptor Mg2+ block extends the action of neurotrophin-3 on synaptic transmission in neonatal rat motoneurons. J Neurophysiol. 2001b;86:123–129. - PubMed
-
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

