Locomotor rhythmogenesis in the isolated rat spinal cord: a phase-coupled set of symmetrical flexion extension oscillators
- PMID: 17569737
- PMCID: PMC2277226
- DOI: 10.1113/jphysiol.2007.133413
Locomotor rhythmogenesis in the isolated rat spinal cord: a phase-coupled set of symmetrical flexion extension oscillators
Abstract
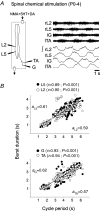
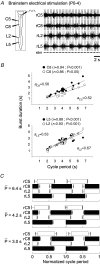
The temporal properties of limb motoneuron bursting underlying quadrupedal locomotion were investigated in isolated spinal cord preparations (without or with brainstem attached) taken from 0 to 4-day-old rats. When activated either with differing combinations of N-methyl-D,L-aspartate, serotonin and dopamine, or by electrical stimulation of the brainstem, the spinal cord generated episodes of fictive locomotion with a constant phase relationship between cervical and lumbar ventral root bursts. Alternation occurred between ipsi- and contra-lateral flexor and extensor motor root bursts, and the cervical and lumbar locomotor networks were always active in a diagonal coordination pattern that corresponded to fictive walking. However, unlike typical locomotion in adult animals in which extensor motoneuron bursts vary more with cycle period than flexor bursts, in the isolated neonatal cord, an increase in fictive locomotor speed was associated with a decrease in the durations of both extensor and flexor bursts, at cervical and lumbar levels. To determine whether this symmetry in flexor/extensor phase durations derived from the absence of sensory feedback that is normally provided from the limbs during intact animal locomotion, EMG recordings were made from hindlimb-attached spinal cords during drug-induced locomotor-like movements. Under these conditions, the duration of extensor muscle bursts increased with cycle period, while flexor burst durations now tended to remain constant. Moreover, after a complete dorsal rhizotomy, this extensor dominant pattern was replaced by flexor and extensor muscle bursts of similar duration. In vivo and in vitro experiments were also conducted on older postnatal (P10-12) rats at an age when body-supported adult-like locomotion occurs. Here again, characteristic extensor-dominated burst patterns observed during intact treadmill locomotion were replaced by symmetrical patterns during fictive locomotion expressed by the chemically activated isolated spinal cord, further indicating that sensory inputs are normally responsible for imposing extensor biasing on otherwise symmetrically alternating extensor/flexor oscillators.
Figures




References
-
- Atsuta Y, Garcia-Rill E, Skinner RD. Electrically induced locomotion in the in vitro brainstem–spinal cord preparation. Brain Res. 1988;470:309–312. - PubMed
-
- Baker LL, Chandler SH, Goldberg LJ. l-dopa-induced locomotor-like activity in ankle flexor and extensor nerves of chronic and acute spinal cats. Exp Neurol. 1984;86:515–526. - PubMed
-
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci. 2001;14:1727–1738. - PubMed
-
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

