Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling
- PMID: 17569887
- PMCID: PMC2680305
- DOI: 10.1161/CIRCRESAHA.107.149047
Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling
Abstract
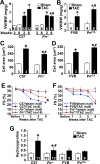
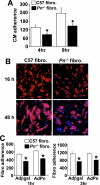
The cardiac extracellular matrix is a dynamic structural support network that is both influenced by, and a regulator of, pathological remodeling and hypertrophic growth. In response to pathologic insults, the adult heart reexpresses the secreted extracellular matrix protein periostin (Pn). Here we show that Pn is critically involved in regulating the cardiac hypertrophic response, interstitial fibrosis, and ventricular remodeling following long-term pressure overload stimulation and myocardial infarction. Mice lacking the gene encoding Pn (Postn) were more prone to ventricular rupture in the first 10 days after a myocardial infarction, but surviving mice showed less fibrosis and better ventricular performance. Pn(-/-) mice also showed less fibrosis and hypertrophy following long-term pressure overload, suggesting an intimate relationship between Pn and the regulation of cardiac remodeling. In contrast, inducible overexpression of Pn in the heart protected mice from rupture following myocardial infarction and induced spontaneous hypertrophy with aging. With respect to a mechanism underlying these alterations, Pn(-/-) hearts showed an altered molecular program in fibroblast function. Indeed, fibroblasts isolated from Pn(-/-) hearts were less effective in adherence to cardiac myocytes and were characterized by a dramatic alteration in global gene expression (7% of all genes). These are the first genetic data detailing the function of Pn in the adult heart as a regulator of cardiac remodeling and hypertrophy.
Figures





Comment in
-
Periostin: more than just an adhesion molecule.Circ Res. 2007 Aug 3;101(3):230-1. doi: 10.1161/CIRCRESAHA.107.159103. Circ Res. 2007. PMID: 17673682 No abstract available.
References
-
- Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. - PubMed
-
- Haldeman GA, Croft JB, Giles WH, Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am Heart J. 1999;137:352–360. - PubMed
-
- Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. - PubMed
-
- Manso AM, Elsherif L, Kang SM, Ross RS. Integrins, membrane-type matrix metalloproteinases and ADAMs: potential implications for cardiac remodeling. Cardiovasc Res. 2006;69:574–584. - PubMed
-
- Deschamps AM, Spinale FG. Matrix modulation and heart failure: new concepts question old beliefs. Curr Opin Cardiol. 2005;20:211–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

