Investigating sacroplasty: technical considerations and finite element analysis of polymethylmethacrylate infusion into cadaveric sacrum
- PMID: 17569952
- PMCID: PMC8134157
- DOI: 10.3174/ajnr.A0500
Investigating sacroplasty: technical considerations and finite element analysis of polymethylmethacrylate infusion into cadaveric sacrum
Abstract
Background and purpose: Sacroplasty is not as routinely performed as vertebroplasty, possibly due to technical challenges and the paucity of data regarding subsequent outcomes. The first goal of the present investigation was to describe a technique for sacroplasty that facilitates safe needle placement and polymethylmethacrylate (PMMA) extrusion. The second goal was to perform finite element analysis (FEA) by using a geometric model of sacral fracture to identify mechanical outcomes of sacroplasty.
Materials and methods: Sacroplasty was performed on fresh pelvis specimens (n=4) under biplane fluoroscopy. Cadavers were imaged via CT before and after sacroplasty and volume rendered to examine needle placement and PMMA extrusion. The volume-rendered CT data were then used to generate geometric models of the intact, fractured, and cement-augmented fractured sacrum for comparison by using FEA.
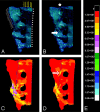
Results: CT data demonstrate that safe injection needle placement and PMMA delivery may be facilitated by orienting the needle parallel to the L5-S1 interspace and ipsilateral sacroiliac joint, then targeting the superolateral sacral ala within an area bounded by a line lateral to the posterior foraminal openings and a line superimposed on the medial edge of the sacroiliac joint. FEA revealed that simulated sacroplasty decreased maximal principal stress at the point of sacral fracture propagation by 83% and fracture gap micromotion by 48%.
Conclusion: Sacral landmarks can be used to place PMMA safely where sacral fractures occur. FEA suggests that sacroplasty may decrease fracture-associated mechanical stress and micromotion, which may contribute to patient reports of decreased pain and increased mobility postsacroplasty.
Figures



References
-
- Lourie H. Spontaneous osteoporotic fracture of the sacrum: an unrecognized syndrome of the elderly. JAMA 1982;248:715–17 - PubMed
-
- Lin J, Lachmann E, Nagler W. Sacral insufficiency fractures: a report of two cases and a review of the literature. J Womens Health Gend Based Med 2001;10:699–705 - PubMed
-
- Grasland A, Pouchot J, Mathieu A, et al. Sacral insufficiency fractures: an easily overlooked cause of back pain in elderly women. Arch Intern Med 1996;156:668–74 - PubMed
-
- Babayev M, Lachmann E, Nagler W. The controversy surrounding sacral insufficiency fractures: to ambulate or not to ambulate? Am J Phys Med Rehabil 2000;79:404–09 - PubMed
-
- Lin JT, Lane JM. Sacral stress fractures. J Womens Health 2003;12:879–88 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
