Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis
- PMID: 17570216
- PMCID: PMC2366898
- DOI: 10.1053/j.gastro.2007.03.101
Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis
Abstract
Background & aims: We studied the role of protease-activated receptor 2 (PAR(2)) and its activating enzymes, trypsins and tryptase, in Clostridium difficile toxin A (TxA)-induced enteritis.
Methods: We injected TxA into ileal loops in PAR(2) or dipeptidyl peptidase I (DPPI) knockout mice or in wild-type mice pretreated with tryptase inhibitors (FUT-175 or MPI-0442352) or soybean trypsin inhibitor. We examined the effect of TxA on expression and activity of PAR(2) and trypsin IV messenger RNA in the ileum and cultured colonocytes. We injected activating peptide (AP), trypsins, tryptase, and p23 in wild-type mice, some pretreated with the neurokinin 1 receptor antagonist SR140333.
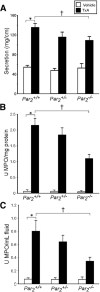
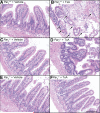
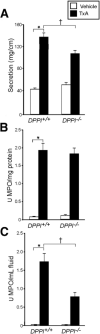
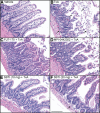
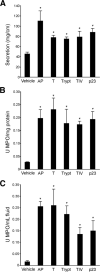
Results: TxA increased fluid secretion, myeloperoxidase activity in fluid and tissue, and histologic damage. PAR(2) deletion decreased TxA-induced ileitis, reduced luminal fluid secretion by 20%, decreased tissue and fluid myeloperoxidase by 50%, and diminished epithelial damage, edema, and neutrophil infiltration. DPPI deletion reduced secretion by 20% and fluid myeloperoxidase by 55%. In wild-type mice, FUT-175 or MPI-0442352 inhibited secretion by 24%-28% and tissue and fluid myeloperoxidase by 31%-71%. Soybean trypsin inhibitor reduced secretion to background levels and tissue myeloperoxidase by up to 50%. TxA increased expression of PAR(2) and trypsin IV in enterocytes and colonocytes and caused a 2-fold increase in Ca(2+) responses to PAR(2) AP. AP, tryptase, and trypsin isozymes (trypsin I/II, trypsin IV, p23) caused ileitis. SR140333 prevented AP-induced ileitis.
Conclusions: PAR(2) and its activators are proinflammatory in TxA-induced enteritis. TxA stimulates existing PAR(2) and up-regulates PAR(2) and activating proteases, and PAR(2) causes inflammation by neurogenic mechanisms.
Figures





Comment in
-
Protease-activated receptor 2 in the intestinal inflammatory response induced by Clostridium difficile toxin A.Gastroenterology. 2007 Jun;132(7):2599-601. doi: 10.1053/j.gastro.2007.04.041. Gastroenterology. 2007. PMID: 17570231 No abstract available.
References
-
- Bunnett NW, Cottrell GS. Protease-activated receptors in gastrointestinal function and disease. In: Lendeckel U, Hooper NM, editors. Proteases in gastrointestinal tissues. Vol. 5. Dordrecht: Springer: 2006. pp. 1–32.
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK043207/DK/NIDDK NIH HHS/United States
- R01 DK047343/DK/NIDDK NIH HHS/United States
- R56 DK043207/DK/NIDDK NIH HHS/United States
- DK43207/DK/NIDDK NIH HHS/United States
- DK52388/DK/NIDDK NIH HHS/United States
- DK41301/DK/NIDDK NIH HHS/United States
- P01 HL024136/HL/NHLBI NIH HHS/United States
- HL024136/HL/NHLBI NIH HHS/United States
- DK47343/DK/NIDDK NIH HHS/United States
- R01 DK057840/DK/NIDDK NIH HHS/United States
- DK57840/DK/NIDDK NIH HHS/United States
- R01 DK039957/DK/NIDDK NIH HHS/United States
- R37 DK039957/DK/NIDDK NIH HHS/United States
- R01 DK052388/DK/NIDDK NIH HHS/United States
- DK39957/DK/NIDDK NIH HHS/United States
- P30 DK041301/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

