Protein kinase C iota: human oncogene, prognostic marker and therapeutic target
- PMID: 17570678
- PMCID: PMC2705893
- DOI: 10.1016/j.phrs.2007.04.015
Protein kinase C iota: human oncogene, prognostic marker and therapeutic target
Abstract
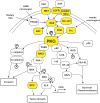
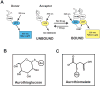
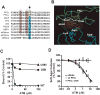
The protein kinase C (PKC) family of serine/threonine kinases has been the subject of intensive study in the field of cancer since their initial discovery as major cellular receptors for the tumor promoting phorbol esters nearly 30 years ago. However, despite these efforts, the search for a direct genetic link between members of the PKC family and human cancer has yielded only circumstantial evidence that any PKC isozyme is a true cancer gene. This situation changed in the past year with the discovery that atypical protein kinase C iota (PKC iota) is a bonafide human oncogene. PKC iota is required for the transformed growth of human cancer cells and the PKC iota gene is the target of tumor-specific gene amplification in multiple forms of human cancer. PKC iota participates in multiple aspects of the transformed phenotype of human cancer cells including transformed growth, invasion and survival. Herein, we review pertinent aspects of atypical PKC structure, function and regulation that relate to the role of these enzymes in oncogenesis. We discuss the evidence that PKC iota is a human oncogene, review mechanisms controlling PKC iota expression in human cancers, and describe the molecular details of PKC iota-mediated oncogenic signaling. We conclude with a discussion of how oncogenic PKC iota signaling has been successfully targeted to identify a novel, mechanism-based therapeutic drug currently entering clinical trials for treatment of human lung cancer. Throughout, we identify key unanswered questions and exciting future avenues of investigation regarding this important oncogenic molecule.
Figures
References
-
- Takai Y, Yamamoto M, Inoue M, Kishimoto A, Nishizuka Y. A proenzyme of cyclic nucleotide-independent protein kinase and its activation by calcium-dependent neutral protease from rat liver. Biochem Biophys Res Commun. 1977;77(2):542–50. - PubMed
-
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–14. - PubMed
-
- Nishizuka Y. The Albert Lasker Medical Awards. The family of protein kinase C for signal transduction. Jama. 1989;262(13):1826–33. - PubMed
-
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. Faseb J. 1995;9(7):484–96. - PubMed
-
- Kikkawa U, Kishimoto A, Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

