Transcellular diapedesis is initiated by invasive podosomes
- PMID: 17570692
- PMCID: PMC2094044
- DOI: 10.1016/j.immuni.2007.04.015
Transcellular diapedesis is initiated by invasive podosomes
Abstract
Diapedesis is critical for immune system function and inflammatory responses. This occurs by migration of blood leukocytes either directly through individual microvascular endothelial cells (the "transcellular" route) or between them (the "paracellular" route). Mechanisms for transcellular pore formation in endothelium remain unknown. Here we demonstrate that lymphocytes used podosomes and extended "invasive podosomes" to palpate the surface of, and ultimately form transcellular pores through, the endothelium. In lymphocytes, these structures were dependent on Src kinase and the actin regulatory protein WASP; inhibition of podosome formation selectively blocked the transcellular route of diapedesis. In endothelium, membrane fusion events dependent on the SNARE-containing membrane fusion complex and intracellular calcium were required for efficient transcellular pore formation in response to podosomes. These findings provide insights into basic mechanisms for leukocyte trafficking and the functions of podosomes.
Figures
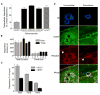

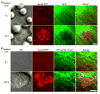
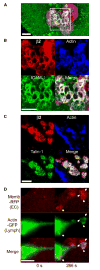

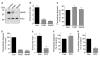
Comment in
-
Leukocyte podosomes sense their way through the endothelium.Immunity. 2007 Jun;26(6):753-5. doi: 10.1016/j.immuni.2007.06.002. Immunity. 2007. PMID: 17582348
References
-
- Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rhodependent pathway. J Immunol. 1999;162:2964–2973. - PubMed
-
- Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. - PubMed
-
- Calle Y, Chou HC, Thrasher AJ, Jones GE. Wiskott-Aldrich syndrome protein and the cytoskeletal dynamics of dendritic cells. J Pathol. 2004;204:460–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

