From morphology to neural information: the electric sense of the skate
- PMID: 17571918
- PMCID: PMC1892606
- DOI: 10.1371/journal.pcbi.0030113
From morphology to neural information: the electric sense of the skate
Abstract
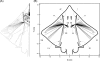
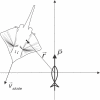
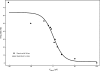
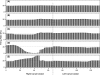
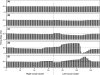
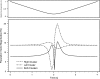
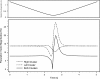
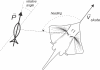
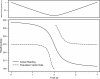
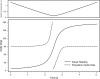
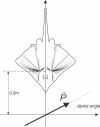
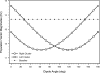
Morphology typically enhances the fidelity of sensory systems. Sharks, skates, and rays have a well-developed electrosense that presents strikingly unique morphologies. Here, we model the dynamics of the peripheral electrosensory system of the skate, a dorsally flattened batoid, moving near an electric dipole source (e.g., a prey organism). We compute the coincident electric signals that develop across an array of the skate's electrosensors, using electrodynamics married to precise morphological measurements of sensor location, infrastructure, and vector projection. Our results demonstrate that skate morphology enhances electrosensory information. Not only could the skate locate prey using a simple population vector algorithm, but its morphology also specifically leads to quick shifts in firing rates that are well-suited to the demonstrated bandwidth of the electrosensory system. Finally, we propose electrophysiology trials to test the modeling scheme.
Conflict of interest statement
Figures





References
-
- Volman SF, Kunishi M. Comparative physiology of sound localization in four species of owls. Brain Behav Evol. 1990;36:196–215. - PubMed
-
- Sturzl W, Kempter R, van Hemmen JL. Theory of arachnid prey localization. Phys Rev Lett. 2000;84:5668–5671. - PubMed
-
- Franosch JM, Sobotka MC, Elepfandt A, van Hemmen JL. Minimal model of prey localization through the lateral-line system. Phys Rev Lett. 2003;91:158101. - PubMed
-
- Zakon HH. The electroreceptive periphery. In: Bullock TH, Heiligenberg W, editors. Electroreception. New York: Wiley; 1986. pp. 103–156.
-
- Nelson ME, MacIver M. Prey capture in the weakly electric fish Apteronotus albifrons: Sensory acquisition strategies and electrosensory consequences. J Exp Biol. 1999;202:1195–1203. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

