Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis
- PMID: 17572669
- PMCID: PMC2680956
- DOI: 10.1038/ncb1609
Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis
Abstract
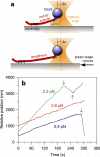
In dividing cells, kinetochores couple chromosomes to the tips of growing and shortening microtubule fibres and tension at the kinetochore-microtubule interface promotes fibre elongation. Tension-dependent microtubule fibre elongation is thought to be essential for coordinating chromosome alignment and separation, but the mechanism underlying this effect is unknown. Using optical tweezers, we applied tension to a model of the kinetochore-microtubule interface composed of the yeast Dam1 complex bound to individual dynamic microtubule tips. Higher tension decreased the likelihood that growing tips would begin to shorten, slowed shortening, and increased the likelihood that shortening tips would resume growth. These effects are similar to the effects of tension on kinetochore-attached microtubule fibres in many cell types, suggesting that we have reconstituted a direct mechanism for microtubule-length control in mitosis.
Figures



References
-
- Koshland DE, Mitchison TJ, Kirschner MW. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature. 1988;331:499–504. - PubMed
-
- Nicklas RB. The forces that move chromosomes in mitosis. Annu Rev Biophys Biophys Chem. 1988;17:431–49. - PubMed
-
- Skibbens RV, Salmon ED. Micromanipulation of chromosomes in mitotic vertebrate tissue cells: tension controls the state of kinetochore movement. Exp Cell Res. 1997;235:314–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

