Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator
- PMID: 17572670
- PMCID: PMC2909385
- DOI: 10.1038/nchembio.2007.4
Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator
Abstract
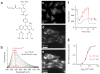
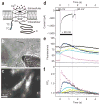
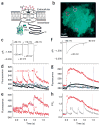
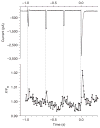
Intracellular Ca(2+) regulates numerous proteins and cellular functions and can vary substantially over submicron and submillisecond scales, so precisely localized fast detection is desirable. We have created a approximately 1-kDa biarsenical Ca(2+) indicator, called Calcium Green FlAsH (CaGF, 1), to probe [Ca(2+)] surrounding genetically targeted proteins. CaGF attached to a tetracysteine motif becomes ten-fold more fluorescent upon binding Ca(2+), with a K(d) of approximately 100 microM, <1-ms kinetics and good Mg(2+) rejection. In HeLa cells expressing tetracysteine-tagged connexin 43, CaGF labels gap junctions and reports Ca(2+) waves after injury. Total internal reflection microscopy of tetracysteine-tagged, CaGF-labeled alpha(1C) L-type calcium channels shows fast-rising depolarization-evoked Ca(2+) transients, whose lateral nonuniformity suggests that the probability of channel opening varies greatly over micron dimensions. With moderate Ca(2+) buffering, these transients decay surprisingly slowly, probably because most of the CaGF signal comes from closed channels feeling Ca(2+) from a tiny minority of clustered open channels. With high Ca(2+) buffering, CaGF signals decay as rapidly as the calcium currents, as expected for submicron Ca(2+) domains immediately surrounding active channels. Thus CaGF can report highly localized, rapid [Ca(2+)] dynamics.
Conflict of interest statement
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at
Figures

Comment in
-
Calcium channels light up.Nat Chem Biol. 2007 Jul;3(7):369-70. doi: 10.1038/nchembio0707-369. Nat Chem Biol. 2007. PMID: 17576420 No abstract available.
References
-
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. - PubMed
-
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. - PubMed
-
- Parnas H, Segel L, Dudel J, Parnas I. Autoreceptors, membrane potential and the regulation of transmitter release. Trends Neurosci. 2000;23:60–68. - PubMed
-
- Llinás R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. - PubMed
Publication types
MeSH terms
Substances
Associated data
- PubChem-Substance/24711513
- PubChem-Substance/24711514
- PubChem-Substance/24711515
- PubChem-Substance/24711516
- PubChem-Substance/24711517
- PubChem-Substance/24711518
- PubChem-Substance/24711519
- PubChem-Substance/24711520
- PubChem-Substance/24711521
- PubChem-Substance/24711522
- PubChem-Substance/24711523
- PubChem-Substance/24711524
- PubChem-Substance/24711525
- PubChem-Substance/24711526
- PubChem-Substance/24711527
- PubChem-Substance/24711528
- PubChem-Substance/24711529
- PubChem-Substance/24711530
- PubChem-Substance/24711531
- PubChem-Substance/24711532
- PubChem-Substance/24711533
- PubChem-Substance/24711534
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

