Viruses as anticancer drugs
- PMID: 17573126
- PMCID: PMC3125087
- DOI: 10.1016/j.tips.2007.05.005
Viruses as anticancer drugs
Abstract
Oncolytic viruses are being developed as anticancer drugs. They propagate selectively in tumor tissue and destroy it without causing excessive damage to normal non-cancerous tissues. When used as drugs, they must meet stringent criteria for safety and efficacy and be amenable to pharmacological study in human subjects. Specificity for neoplastic tissue is the key to safety, and this goal can be achieved through a variety of ingenious virus-engineering strategies. Antiviral immunity remains a significant barrier to the clinical efficacy of oncolytic viruses but this is being addressed by using novel immune-evasive delivery strategies and immunosuppressive drugs. Noninvasive pharmacokinetic monitoring is facilitated by engineering marker genes into the viral genome. Clinical data on the pharmacokinetics of oncolytic viruses will be the key to accelerating their development and approval as effective anticancer drugs. This review introduces concepts relevant to the use of viruses as anticancer drugs, emphasizing targeting mechanisms as well as safety and efficacy issues that are currently limiting their clinical success.
Figures
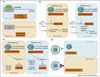

References
-
- Condit RC. Principles of virology. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; 2001. pp. 19–51.
-
- Harrison SC. Principles of virus structure. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams and Wilkins; 2001. pp. 53–85.
-
- Parato KA, et al. Recent progress in the battle between oncolytic viruses and tumours. Nat. Rev. Cancer. 2005;5:965–976. - PubMed
-
- Kelly E, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. - PubMed
-
- Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets. 2007;7:141–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

