Flavonoids affect actin functions in cytoplasm and nucleus
- PMID: 17573428
- PMCID: PMC1989700
- DOI: 10.1529/biophysj.107.107813
Flavonoids affect actin functions in cytoplasm and nucleus
Abstract
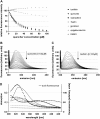
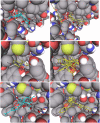
Based on the identification of actin as a target protein for the flavonol quercetin, the binding affinities of quercetin and structurally related flavonoids were determined by flavonoid-dependent quenching of tryptophan fluorescence from actin. Irrespective of differences in the hydroxyl pattern, similar Kd values in the 20 microM range were observed for six flavonoids encompassing members of the flavonol, isoflavone, flavanone, and flavane group. The potential biological relevance of the flavonoid/actin interaction in the cytoplasm and the nucleus was addressed using an actin polymerization and a transcription assay, respectively. In contrast to the similar binding affinities, the flavonoids exert distinct and partially opposing biological effects: although flavonols inhibit actin functions, the structurally related flavane epigallocatechin promotes actin activity in both test systems. Infrared spectroscopic evidence reveals flavonoid-specific conformational changes in actin which may mediate the different biological effects. Docking studies provide models of flavonoid binding to the known small molecule-binding sites in actin. Among these, the mostly hydrophobic tetramethylrhodamine-binding site is a prime candidate for flavonoid binding and rationalizes the high efficiency of quenching of the two closely located fluorescent tryptophans. The experimental and theoretical data consistently indicate the importance of hydrophobic, rather than H-bond-mediated, actin-flavonoid interactions. Depending on the rigidity of the flavonoid structures, different functionally relevant conformational changes are evoked through an induced fit.
Figures




References
-
- Middleton, E., C. Kandaswami, and T. C. Theoharides. 2000. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52:673–751. - PubMed
-
- Knekt, P., J. Kumpulainen, R. Jarvinen, H. Rissanen, M. Heliovaara, A. Reunanen, T. Hakulinen, and A. Aromaa. 2002. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 76:560–568. - PubMed
-
- Manach, C., A. Scalbert, C. Morand, C. Remesy, and L. Jimenez. 2004. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 79:727–747. - PubMed
-
- Nichenametla, S. N., T. G. Taruscio, D. L. Barney, and J. H. Exon. 2006. A review of the effects and mechanisms of polyphenolics in cancer. Crit. Rev. Food Sci. Nutr. 46:161–183. - PubMed
-
- Chen, D., K. G. Daniel, M. S. Chen, D. J. Kuhn, K. R. Landis-Piwowar, and Q. P. Dou. 2005. Dietary flavonoids as proteasome inhibitors and apoptosis inducers in human leukemia cells. Biochem. Pharmacol. 69:1421–1432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

