The N-terminal domain of OmpATb is required for membrane translocation and pore-forming activity in mycobacteria
- PMID: 17573469
- PMCID: PMC1951930
- DOI: 10.1128/JB.00509-07
The N-terminal domain of OmpATb is required for membrane translocation and pore-forming activity in mycobacteria
Abstract
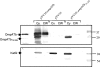
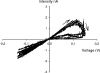
OmpATb is the prototype of a new family of porins in Mycobacterium tuberculosis and Mycobacterium bovis BCG. Although the pore-forming activity of this protein has been clearly established by using recombinant protein produced in Escherichia coli, characterization of the native porin has been hampered by the scarce amount of protein present in the M. tuberculosis detergent extracts. To this aim, we have developed a protocol to overproduce and obtain high yields of OmpATb in both Mycobacterium smegmatis and M. bovis BCG. The protein could be extracted and purified from the cell wall fraction and subsequently used for analysis of the pore-forming activity in multichannel and single-channel conductance experiments. Our results indicate that OmpATb produced in mycobacteria presents an average conductance value of 1,600+/-100 pS, slightly higher than that of OmpATb produced in E. coli, suggesting the occurrence of OmpATb in a highly ordered organization within the mycobacterial cell wall. In contrast to OmpATb, a truncated form lacking the first 72 amino acids (OmpATb73-326) was essentially found in the cytosol and was not active in planar lipid bilayers. This suggested that the N-terminal domain of OmpATb could participate in targeting of OmpATb to the cell wall. This was further confirmed by analyzing M. smegmatis clones expressing a chimeric protein consisting of a fusion between the N-terminal domain of OmpATb and the E. coli PhoA reporter. The present study shows for the first time that the N terminus of OmpATb is required for targeting the porin to the cell wall and also appears to be essential for its pore-forming activity.
Figures





References
-
- Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. - PubMed
-
- Faller, M., M. Niederweis, and G. E. Schulz. 2004. The structure of a mycobacterial outer-membrane channel. Science 303:1189-1192. - PubMed
-
- Heinz, C., H. Engelhardt, and M. Niederweis. 2003. The core of the tetrameric mycobacterial porin MspA is an extremely stable beta-sheet domain. J. Biol. Chem. 278:8678-8685. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

