Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock
- PMID: 17573521
- PMCID: PMC2703721
- DOI: 10.1378/chest.07-0234
Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock
Abstract
Objective: To determine the clinical effectiveness of implementing early goal-directed therapy (EGDT) as a routine protocol in the emergency department (ED).
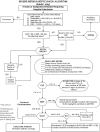
Methods: Prospective interventional study conducted over 2 years at an urban ED. Inclusion criteria included suspected infection, criteria for systemic inflammation, and either systolic BP < 90 mm Hg after a fluid bolus or lactate concentration >/= 4 mol/L. Exclusion criteria were age < 18 years, contraindication to a chest central venous catheter, and need for immediate surgery. We prospectively recorded preintervention clinical and mortality data on consecutive, eligible patients for 1 year when treatment was at the discretion of board-certified emergency physicians. We then implemented an EGDT protocol (the intervention) and recorded clinical data and mortality rates for 1 year. Prior to the first year, we defined a 33% relative reduction in mortality (relative mortality reduction that was found in the original EGDT trial) to indicate clinical effectiveness of the intervention.
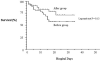
Results: We enrolled 79 patients in the preintervention year and 77 patients in the postintervention year. Compared with the preintervention year, patients in the postintervention year received significantly greater crystalloid volume (2.54 L vs 4.66 L, p < 0.001) and frequency of vasopressor infusion (34% vs 69%, p < 0.001) during the initial resuscitation. In-hospital mortality was 21 of 79 patients (27%) before intervention, compared with 14 of 77 patients (18%) after intervention (absolute difference, - 9%; 95% confidence interval, + 5 to - 21%).
Conclusions: Implementation of EGDT in our ED was associated with a 9% absolute (33% relative) mortality reduction. Our data provide external validation of the clinical effectiveness of EGDT to treat sepsis and septic shock in the ED.
Figures

Comment in
-
Resource utilization in patients undergoing early goal-directed therapy for severe sepsis and septic shock.Chest. 2008 Jan;133(1):315; author reply 316. doi: 10.1378/chest.07-2206. Chest. 2008. PMID: 18187765 No abstract available.
-
Clinical effectiveness and early goal-directed therapy for severe sepsis and septic shock.Chest. 2008 Feb;133(2):584; author reply 584-5. doi: 10.1378/chest.07-1916. Chest. 2008. PMID: 18252930 No abstract available.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Kochanek KD, Smith B. National vital statistics report. In: Centers for Disease Control and Prevention, editor. Deaths: preliminary data for 2002. No 13. Vol 52. US Department of Health and Human Services, National Vital Statistics Report; Hyattsville, MD: 2004. pp. 1–32. - PubMed
-
- Strehlow MC, Emond SD, Shapiro NI, et al. National study of emergency department visits for sepsis, 1992 to 2001. Ann Emerg Med. 2006;48:326–331. - PubMed
-
- Centers for Disease Control and Prevention Current trends increase in national hospital discharge survey rates for septicemia - United States, 1979-1987. MMRW Morb Mortal Wkly Rep. 1991;39:31–34. - PubMed
-
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

