Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination
- PMID: 17573530
- PMCID: PMC1904174
- DOI: 10.1073/pnas.0703766104
Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination
Abstract
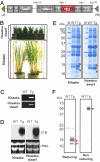
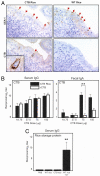
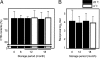
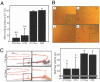
Capable of inducing antigen-specific immune responses in both systemic and mucosal compartments without the use of syringe and needle, mucosal vaccination is considered ideal for the global control of infectious diseases. In this study, we developed a rice-based oral vaccine expressing cholera toxin B subunit (CTB) under the control of the endosperm-specific expression promoter 2.3-kb glutelin GluB-1 with codon usage optimization for expression in rice seed. An average of 30 mug of CTB per seed was stored in the protein bodies, which are storage organelles in rice. When mucosally fed, rice seeds expressing CTB were taken up by the M cells covering the Peyer's patches and induced CTB-specific serum IgG and mucosal IgA antibodies with neutralizing activity. When expressed in rice, CTB was protected from pepsin digestion in vitro. Rice-expressed CTB also remained stable and thus maintained immunogenicity at room temperature for >1.5 years, meaning that antigen-specific mucosal immune responses were induced at much lower doses than were necessary with purified recombinant CTB. Because they require neither refrigeration (cold-chain management) nor a needle, these rice-based mucosal vaccines offer a highly practical and cost-effective strategy for orally vaccinating large populations against mucosal infections, including those that may result from an act of bioterrorism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Vaccines are for dinner.Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):10757-8. doi: 10.1073/pnas.0704516104. Epub 2007 Jun 20. Proc Natl Acad Sci U S A. 2007. PMID: 17581867 Free PMC article. Review. No abstract available.
References
-
- Watanabe I, Hagiwara Y, Kadowaki SE, Yoshikawa T, Komase K, Aizawa C, Kiyono H, Takeda Y, McGhee JR, Chiba J, et al. Vaccine. 2002;20:3443–3455. - PubMed
-
- Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, et al. Nat Med. 2001;7:1320–1326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

