Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis
- PMID: 17573535
- PMCID: PMC1955726
- DOI: 10.1105/tpc.107.051383
Arabidopsis cytochrome P450 monooxygenase 71A13 catalyzes the conversion of indole-3-acetaldoxime in camalexin synthesis
Abstract
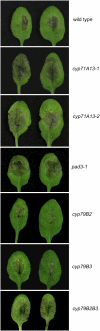
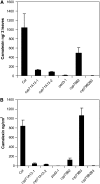
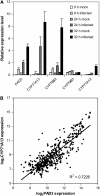
Camalexin (3-thiazol-2-yl-indole) is an indole alkaloid phytoalexin produced by Arabidopsis thaliana that is thought to be important for resistance to necrotrophic fungal pathogens, such as Alternaria brassicicola and Botrytis cinerea. It is produced from Trp, which is converted to indole acetaldoxime (IAOx) by the action of cytochrome P450 monooxygenases CYP79B2 and CYP79B3. The remaining biosynthetic steps are unknown except for the last step, which is conversion of dihydrocamalexic acid to camalexin by CYP71B15 (PAD3). This article reports characterization of CYP71A13. Plants carrying cyp71A13 mutations produce greatly reduced amounts of camalexin after infection by Pseudomonas syringae or A. brassicicola and are susceptible to A. brassicicola, as are pad3 and cyp79B2 cyp79B3 mutants. Expression levels of CYP71A13 and PAD3 are coregulated. CYP71A13 expressed in Escherichia coli converted IAOx to indole-3-acetonitrile (IAN). Expression of CYP79B2 and CYP71A13 in Nicotiana benthamiana resulted in conversion of Trp to IAN. Exogenously supplied IAN restored camalexin production in cyp71A13 mutant plants. Together, these results lead to the conclusion that CYP71A13 catalyzes the conversion of IAOx to IAN in camalexin synthesis and provide further support for the role of camalexin in resistance to A. brassicicola.
Figures
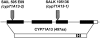





References
-
- Alonso, J.M., Hirayama, T., Roman, G., Nourizadeh, S., and Ecker, J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284 2148–2152. - PubMed
-
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657. - PubMed
-
- Bak, S., Kahn, R., Nielsen, H., Møller, B., and Halkier, B. (1998. b). Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol. Biol. 36 393–405. - PubMed
-
- Bak, S., Nielsen, H., and Halkier, B. (1998. a). The presence of CYP79 homologues in glucosinolate-producing plants shows evolutionary conservation of the enzymes in the conversion of amino acid to aldoxime in the biosynthesis of cyanogenic glucosides and glucosinolates. Plant Mol. Biol. 38 725–734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

