Topographical and laminar distribution of cortical input to the monkey entorhinal cortex
- PMID: 17573826
- PMCID: PMC2375768
- DOI: 10.1111/j.1469-7580.2007.00764.x
Topographical and laminar distribution of cortical input to the monkey entorhinal cortex
Abstract
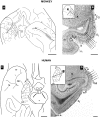
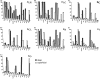
Hippocampal formation plays a prominent role in episodic memory formation and consolidation. It is likely that episodic memory representations are constructed from cortical information that is mostly funnelled through the entorhinal cortex to the hippocampus. The entorhinal cortex returns processed information to the neocortex. Retrograde tracing studies have shown that neocortical afferents to the entorhinal cortex originate almost exclusively in polymodal association cortical areas. However, the use of retrograde studies does not address the question of the laminar and topographical distribution of cortical projections within the entorhinal cortex. We examined material from 60 Macaca fascicularis monkeys in which cortical deposits of either (3)H-amino acids or biotinylated dextran-amine as anterograde tracers were made into different cortical areas (the frontal, cingulate, temporal and parietal cortices). The various cortical inputs to the entorhinal cortex present a heterogeneous topographical distribution. Some projections terminate throughout the entorhinal cortex (afferents from medial area 13 and posterior parahippocampal cortex), while others have more limited termination, with emphasis either rostrally (lateral orbitofrontal cortex, agranular insular cortex, anterior cingulate cortex, perirhinal cortex, unimodal visual association cortex), intermediate (upper bank of the superior temporal sulcus, unimodal auditory association cortex) or caudally (parietal and retrosplenial cortices). Many of these inputs overlap, particularly within the rostrolateral portion of the entorhinal cortex. Some projections were directed mainly to superficial layers (I-III) while others were heavier to deep layers (V-VI) although areas of dense projections typically spanned all layers. A primary report will provide a detailed analysis of the regional and laminar organization of these projections. Here we provide a general overview of these projections in relation to the known neuroanatomy of the entorhinal cortex.
Figures






References
-
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol. 1980;189:573–591. - PubMed
-
- Amaral DG, Insausti R, Cowan WM. Evidence for a direct projection from the superior temporal gyrus to the entorhinal cortex in the monkey. Brain Res. 1983;275:263–277. - PubMed
-
- Amaral DG, Insausti R, Cowan WM. The commissural connections of the monkey hippocampal formation. J Comp Neurol. 1984;224:307–336. - PubMed
-
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey, I. Cytoarchitectonic organization. J Comp Neurol. 1987;264:326–355. - PubMed
-
- von Bonin G, Bailey P. The Neocortex of Macaca mulatta. Urbana, IL: University of Illinois Press; 1947.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

