The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome
- PMID: 17574010
- PMCID: PMC2094733
- DOI: 10.1016/j.beem.2007.03.009
The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome
Abstract
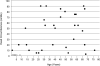
Thyroid hormone is essential for the proper development and function of the brain. The active form of thyroid hormone is T(3), which binds to nuclear receptors. Recently, a transporter specific for T(3), MCT8 (monocarboxylate transporter 8) was identified. MCT8 is highly expressed in liver and brain. The gene is located in Xq13 and mutations in MCT8 are responsible for an X-linked condition, Allan-Herndon-Dudley syndrome (AHDS). This syndrome is characterized by congenital hypotonia that progresses to spasticity with severe psychomotor delays. Affected males also present with muscle hypoplasia, generalized muscle weakness, and limited speech. Importantly, these patients have elevated serum levels of free T(3), low to below normal serum levels of free T(4), and levels of thyroid stimulating hormone that are within the normal range. This constellation of measurements of thyroid function enables quick screening for AHDS in males presenting with cognitive impairment, congenital hypotonia, and generalized muscle weakness.
Figures


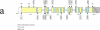
 indicates an insertion of an amino acid (3 bp); – indicates the deletion of the indicated amino acid. Modified from Figure 2 of ref .
indicates an insertion of an amino acid (3 bp); – indicates the deletion of the indicated amino acid. Modified from Figure 2 of ref .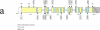
 indicates an insertion of an amino acid (3 bp); – indicates the deletion of the indicated amino acid. Modified from Figure 2 of ref .
indicates an insertion of an amino acid (3 bp); – indicates the deletion of the indicated amino acid. Modified from Figure 2 of ref .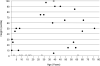









References
-
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Role of thyroid hormone during early brain development. Eur J Endocrinol. 2004;151(Suppl 3):U25–U37. - PubMed
-
- Bianco AC, Salvatore D, Gereben B, et al. Biochemistry, cellular and molecular biology, and physiological roles of iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. - PubMed
-
- Hennemann G, Docter R, Friesema EC, et al. Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr Rev. 2001;22:451–476. - PubMed
-
- Friesema EC, Ganguly S, Abdalla A, et al. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter 8 as a specific thyroid hormone transporter. J Biol Chem. 2003;278:40126–40135. - PubMed
-
- Fliers E, Alkemade A, Wiersinga WM, Swaab DF. Hypothalamic thyroid hormone feedback in health and disease. Progress in Brain Research. 2006;153:189–207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

