A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency
- PMID: 17574023
- PMCID: PMC1974882
- DOI: 10.1016/j.cell.2007.05.014
A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency
Abstract
Myeloproliferative syndromes (MPS) are a heterogeneous subclass of nonlymphoid hematopoietic neoplasms which are considered to be intrinsic to hematopoietic cells. The causes of MPS are largely unknown. Here, we demonstrate that mice deficient for retinoic acid receptor gamma (RARgamma), develop MPS induced solely by the RARgamma-deficient microenvironment. RARgamma(-/-) mice had significantly increased granulocyte/macrophage progenitors and granulocytes in bone marrow (BM), peripheral blood, and spleen. The MPS phenotype continued for the lifespan of the mice and was more pronounced in older mice. Unexpectedly, transplant studies revealed this disease was not intrinsic to the hematopoietic cells. BM from wild-type mice transplanted into mice with an RARgamma(-/-) microenvironment rapidly developed the MPS, which was partially caused by significantly elevated TNFalpha in RARgamma(-/-) mice. These data show that loss of RARgamma results in a nonhematopoietic cell-intrinsic MPS, revealing the capability of the microenvironment to be the sole cause of hematopoietic disorders.
Figures


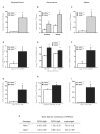

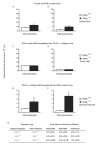
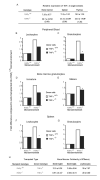
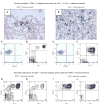
Comment in
-
Disrupting the stem cell niche: good seeds in bad soil.Cell. 2007 Jun 15;129(6):1045-7. doi: 10.1016/j.cell.2007.05.053. Cell. 2007. PMID: 17574018
References
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. - PubMed
-
- Araki T, Mohi MG, Ismat FA, Bronson RT, Williams IR, Kutok JL, Yang W, Pao LI, Gilliland DG, Epstein JA, Neel BG. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10:849–857. - PubMed
-
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. - PubMed
-
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

