Inhibition of hepatitis C virus p7 membrane channels in a liposome-based assay system
- PMID: 17574688
- PMCID: PMC7615709
- DOI: 10.1016/j.antiviral.2007.05.001
Inhibition of hepatitis C virus p7 membrane channels in a liposome-based assay system
Abstract
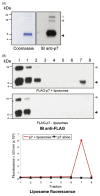
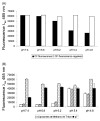
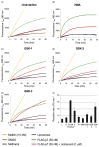
Chemotherapy for patients chronically infected with hepatitis C virus (HCV) is ineffective in over 50% of cases, generating a high demand for new drug targets. The p7 protein of HCV displays membrane channel activity in vitro and is essential for replication in vivo though its precise role in the virus life cycle is unknown. p7 channel activity can be specifically inhibited by several classes of compounds, making this protein an attractive candidate for drug development, though techniques used to date in characterising this protein are unsuited to compound library screening. Here we describe an assay for the channel forming ability of p7 based on the release of a fluorescent indicator from liposomes. We show that recombinant p7 from genotype 1b HCV causes a dose-dependent release of dye when mixed with liposomes and that this property is enhanced at acidic pH. We demonstrate that this activity is due to the formation of a size-selective pore rather than non-specific disruption of liposomes and that activity can be blocked by amantadine and several other compounds, validating it as a measure of p7 channel function. This system provides the first convenient in vitro assay for exploiting p7 as a therapeutic target.
Figures



Similar articles
-
Determinants of hepatitis C virus p7 ion channel function and drug sensitivity identified in vitro.J Virol. 2009 Aug;83(16):7970-81. doi: 10.1128/JVI.00521-09. Epub 2009 Jun 3. J Virol. 2009. PMID: 19493992 Free PMC article.
-
A conserved basic loop in hepatitis C virus p7 protein is required for amantadine-sensitive ion channel activity in mammalian cells but is dispensable for localization to mitochondria.J Gen Virol. 2004 Feb;85(Pt 2):451-461. doi: 10.1099/vir.0.19634-0. J Gen Virol. 2004. PMID: 14769903
-
Ion Channel Function and Cross-Species Determinants in Viral Assembly of Nonprimate Hepacivirus p7.J Virol. 2016 Apr 29;90(10):5075-5089. doi: 10.1128/JVI.00132-16. Print 2016 May 15. J Virol. 2016. PMID: 26962224 Free PMC article.
-
Hepatitis C virus p7: molecular function and importance in hepatitis C virus life cycle and potential antiviral target.Liver Int. 2011 May;31(5):606-17. doi: 10.1111/j.1478-3231.2010.02442.x. Epub 2011 Jan 24. Liver Int. 2011. PMID: 21457434 Review.
-
Structural and Functional Properties of the Hepatitis C Virus p7 Viroporin.Viruses. 2015 Aug 6;7(8):4461-81. doi: 10.3390/v7082826. Viruses. 2015. PMID: 26258788 Free PMC article. Review.
Cited by
-
NMR studies of p7 protein from hepatitis C virus.Eur Biophys J. 2010 Jun;39(7):1097-104. doi: 10.1007/s00249-009-0533-y. Epub 2009 Sep 2. Eur Biophys J. 2010. PMID: 19727701 Free PMC article.
-
Viroporins and inflammasomes: A key to understand virus-induced inflammation.Int J Biochem Cell Biol. 2020 May;122:105738. doi: 10.1016/j.biocel.2020.105738. Epub 2020 Mar 7. Int J Biochem Cell Biol. 2020. PMID: 32156572 Free PMC article. Review.
-
Intracellular proton conductance of the hepatitis C virus p7 protein and its contribution to infectious virus production.PLoS Pathog. 2010 Sep 2;6(9):e1001087. doi: 10.1371/journal.ppat.1001087. PLoS Pathog. 2010. PMID: 20824094 Free PMC article.
-
High-risk human papillomavirus E5 oncoprotein displays channel-forming activity sensitive to small-molecule inhibitors.J Virol. 2012 May;86(9):5341-51. doi: 10.1128/JVI.06243-11. Epub 2012 Feb 22. J Virol. 2012. PMID: 22357280 Free PMC article.
-
The elusive function of the hepatitis C virus p7 protein.Virology. 2014 Aug;462-463:377-87. doi: 10.1016/j.virol.2014.04.018. Epub 2014 Jul 4. Virology. 2014. PMID: 25001174 Free PMC article. Review.
References
-
- Agirre A, Barco A, Carrasco L, Nieva JL. Viroporin-mediated membrane permeabilization. Pore formation by nonstructural poliovirus 2B protein. J Biol Chem. 2002;277:40434–40441. - PubMed
-
- Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, Scarselli E, Cortese R, Nicosia A, Cosset FL. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624–41630. - PubMed
-
- Choo QL, Kuo G, Weiner A, Wang KS, Overby L, Bradley D, Houghton M. Identification of the major, parenteral non-A, non-B hepatitis agent (hepatitis C virus) using a recombinant cDNA approach. Semin Liver Dis. 1992;12:279–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

