Demethylation of promoter C region of estrogen receptor alpha gene is correlated with its enhanced expression in estrogen-ablation resistant MCF-7 cells
- PMID: 17574841
- PMCID: PMC2641007
- DOI: 10.1016/j.jsbmb.2006.12.104
Demethylation of promoter C region of estrogen receptor alpha gene is correlated with its enhanced expression in estrogen-ablation resistant MCF-7 cells
Abstract
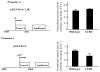
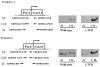
Long-term estrogen deprivation (LTED) MCF-7 cells showing estrogen-independent growth, express estrogen receptor (ER) alpha at a much higher level than wild-type MCF-7 cells. Enhanced expression of ERalpha associated with partial localization of ERalpha to the plasma membranes in LTED cells is thought to be an important step for acquisition of estrogen-ablation resistance. In this study, we compared the regulation of ERalpha gene expression between wild type and LTED cells, examining the usage of the promoters A and C as well as their methylation status. We found that transcription from the promoter C was drastically enhanced in LTED cells, compared with that in wild-type cells. Furthermore, the promoter C region was highly unmethylated in LTED cells, but partially methylated in wild-type cells. Our findings imply that demethylation of promoter C region in the ERalpha gene is in part responsible for the enhanced expression of ERalpha gene in LTED cells.
Figures





References
-
- Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5(3):207–213. - PubMed
-
- Yue W, Wang JP, Li Y, Bocchinfuso WP, Korach KS, Devanesan PD, Rogan E, Cavalieri E, Santen RJ. Tamoxifen versus aromatase inhibitors for breast cancer prevention. Clin Cancer Res. 2005;11(2):925–930. - PubMed
-
- Santen RJ, Harvey HA. Use of aromatase inhibitors in breast carcinoma. Endocr Relat Cancer. 1999;6(1):75–92. - PubMed
-
- Santen RJ, Manni A, Harvey HA, Redmond C. Endocrine treatment of breast cancer in woman. Endocr Rev. 1990;11(2):221–265. - PubMed
-
- Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995;80(10):2918–2925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

